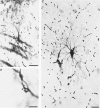
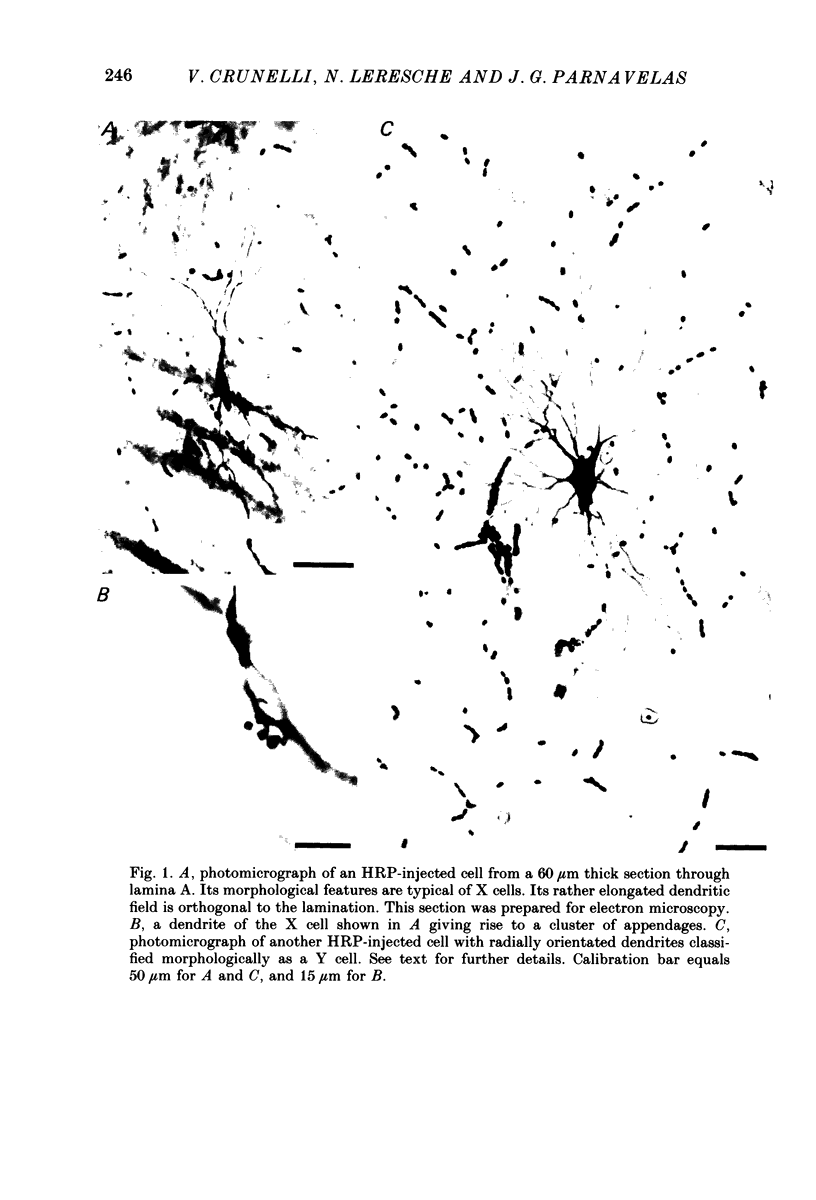
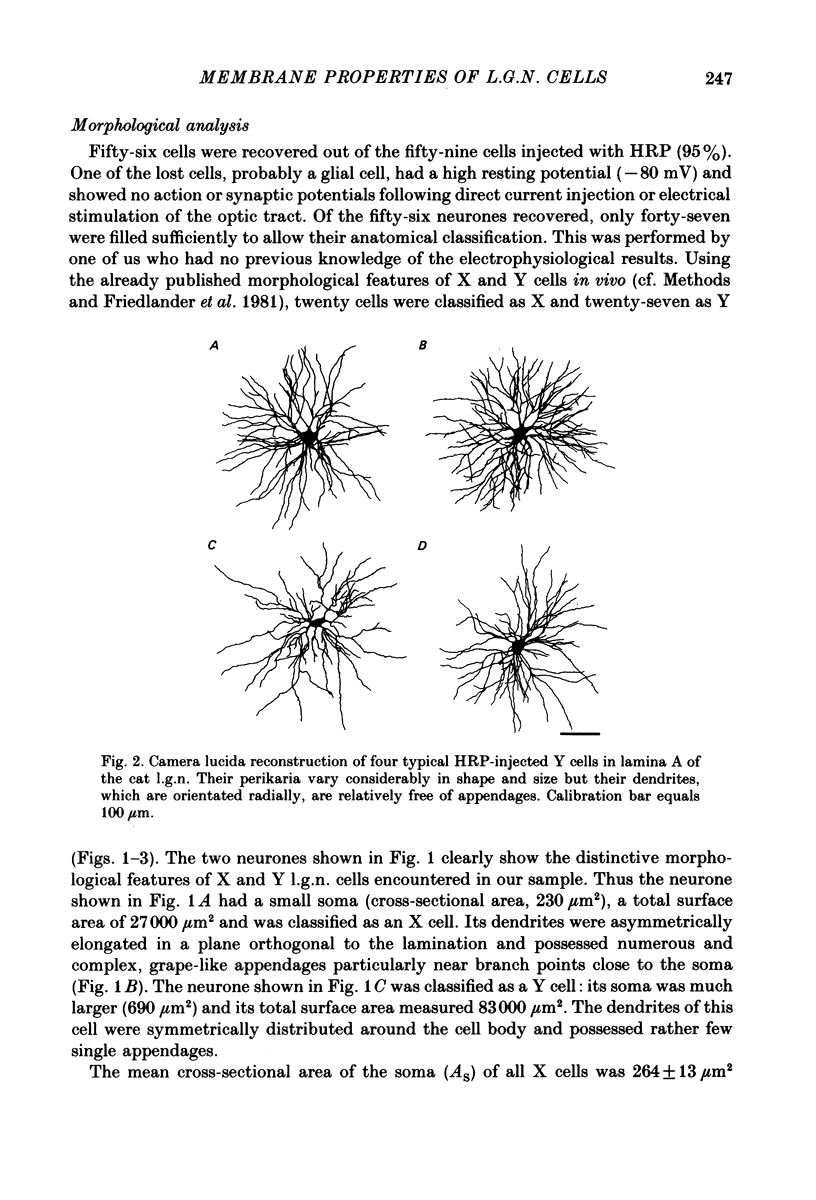
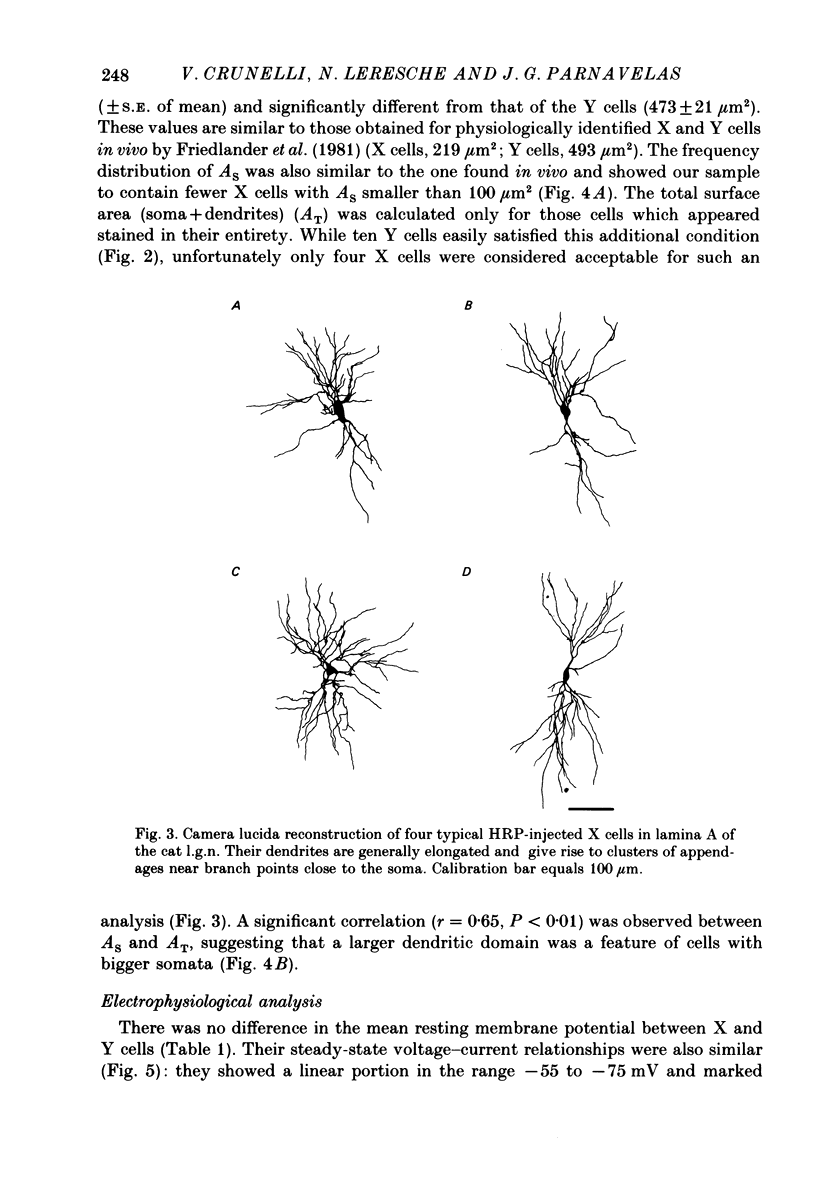
Abstract
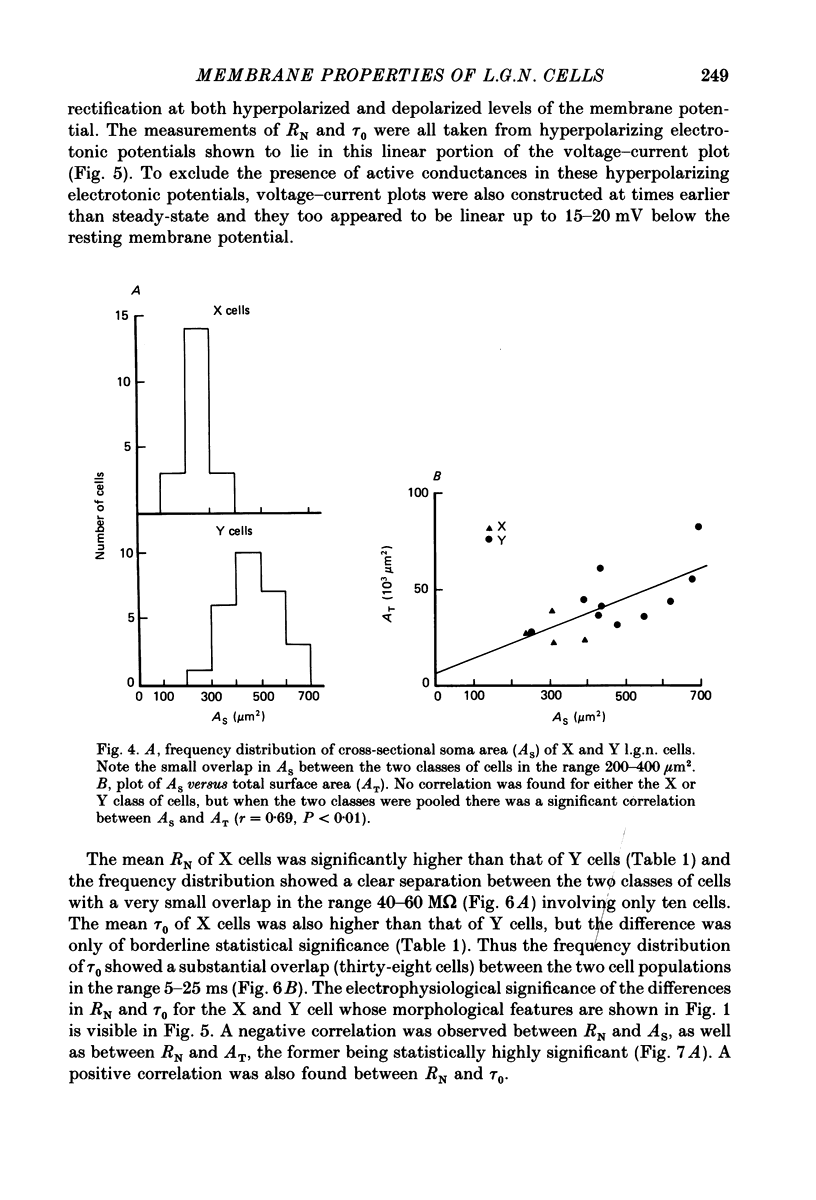
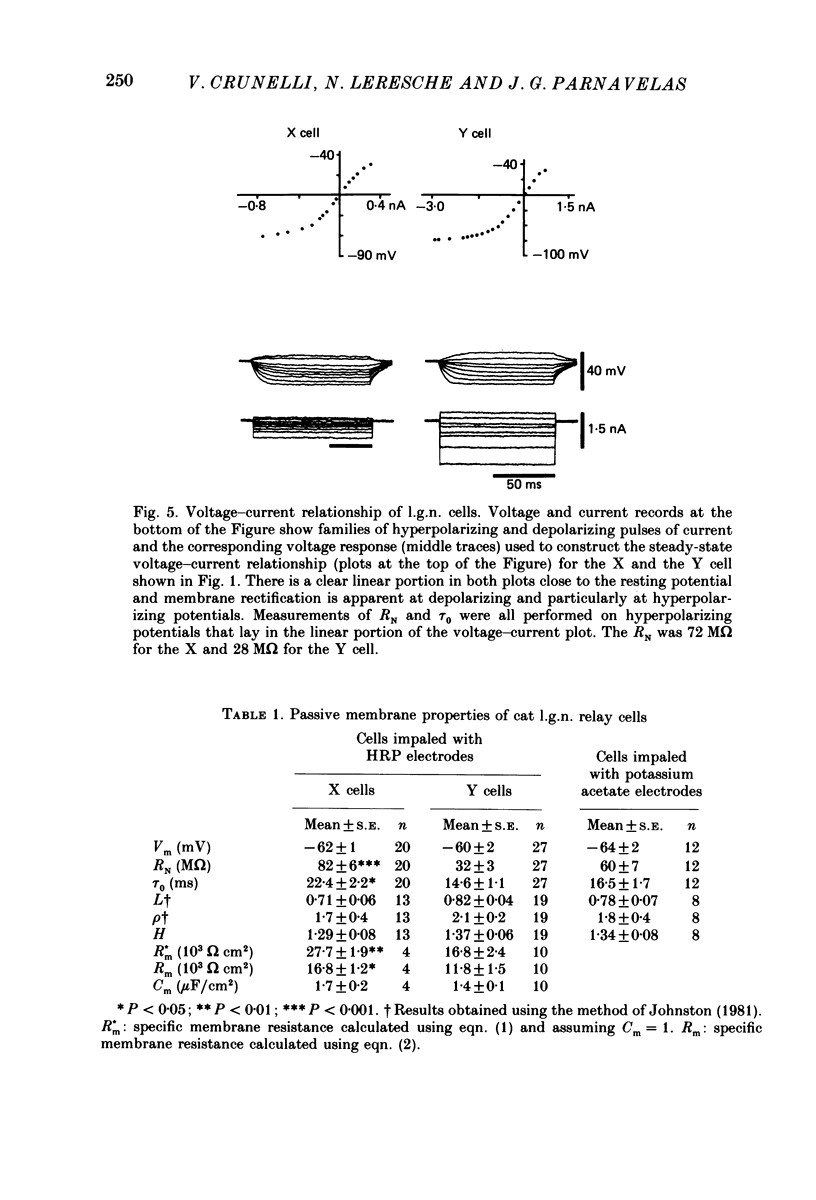
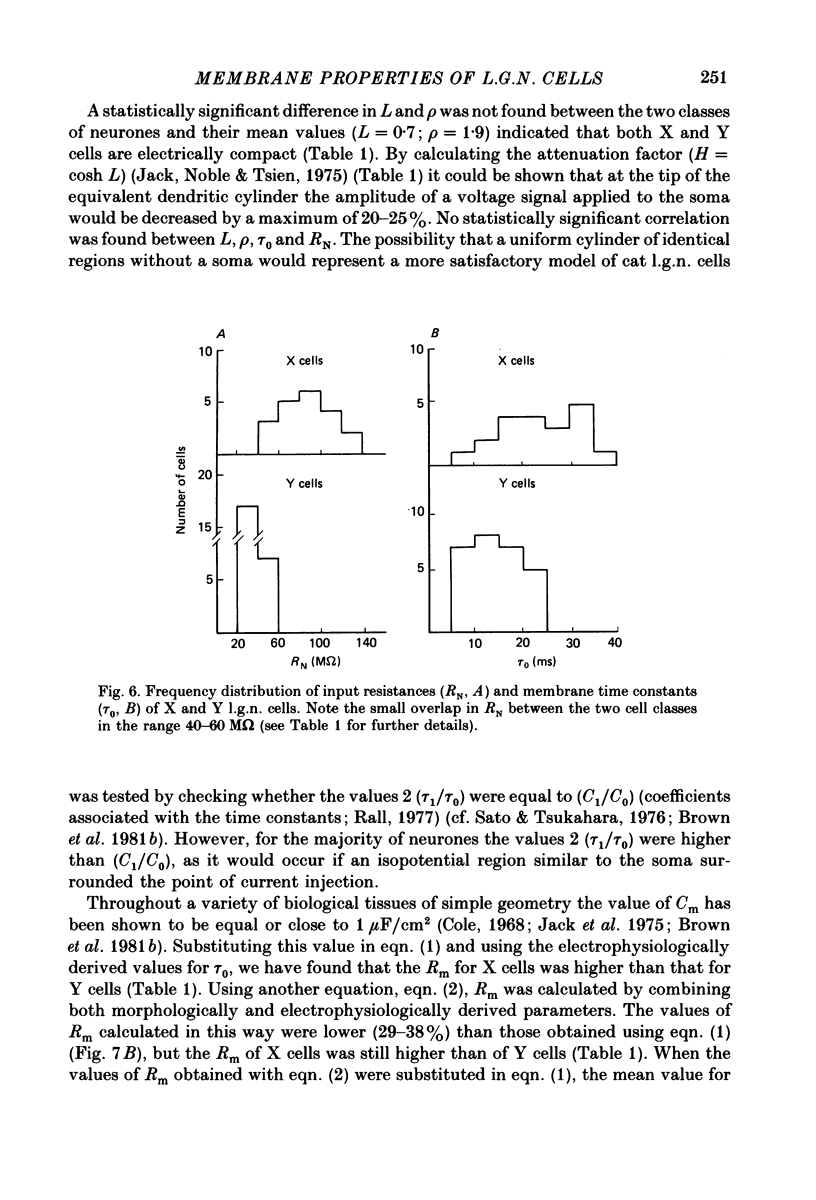
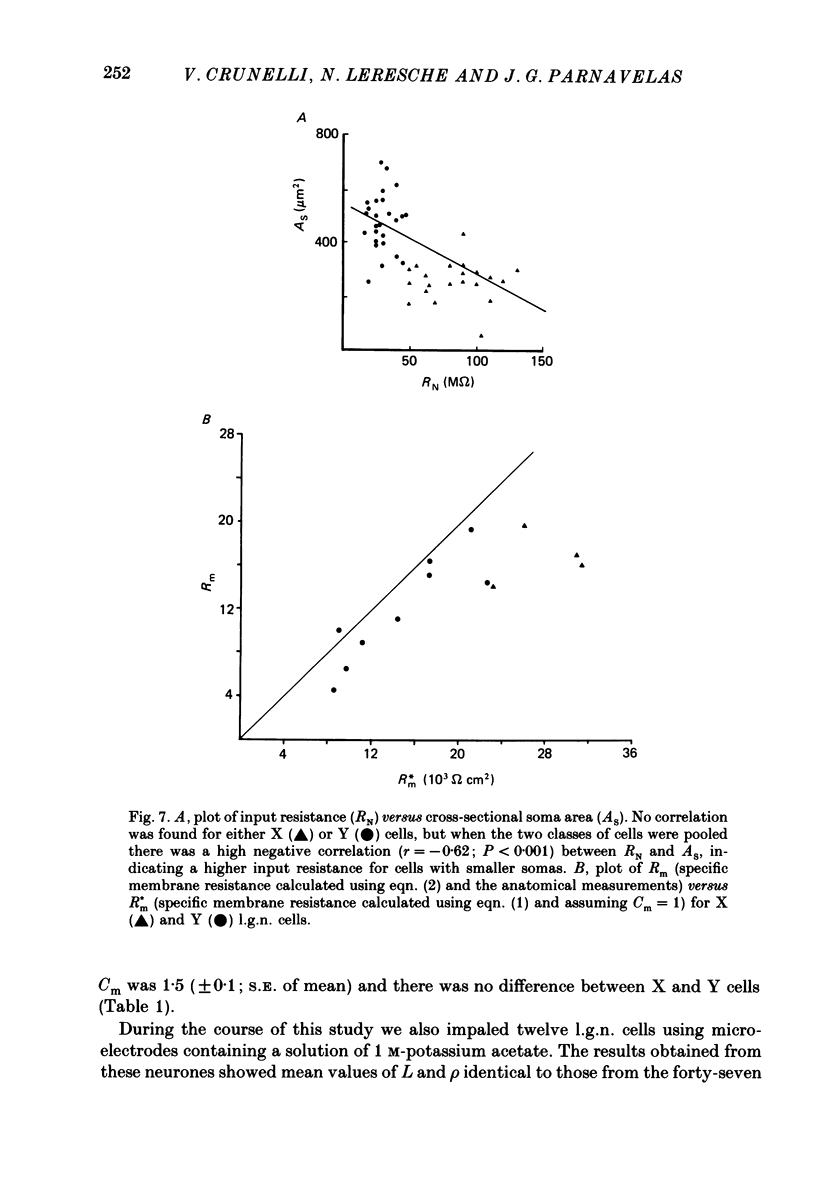
1. The membrane properties and the electrotonic features of cells in lamina A of the cat dorsal lateral geniculate nucleus (l.g.n.) were studied using an in vitro slice preparation. 2. Following intrasomatic injection of horseradish peroxidase (HRP) each neurone was classified as an X (n = 20) or a Y (n = 27) cell on the basis of its morphology. For both classes, the frequency distribution of soma area was similar to that reported in vivo where the identification of X and Y cells in lamina A of the cat l.g.n. was based on physiological criteria. 3. No difference was observed in the mean resting membrane potential between the two classes of cells. However, the input resistance (RN) of X cells was greater (82 M omega) and their membrane time constant (tau 0) longer (22 ms) than of Y cells (RN, 32 M omega; tau 0, 15 ms). 4. Using a simple neuronal model, the calculated electrotonic length (L) and the dendritic to somatic conductance ratio (rho) were similar for the two classes of cells. The mean value of L (0.7) and rho (1.9) suggests that both X and Y cells are electrically compact. 5. The specific membrane resistance (Rm, 28,000 omega cm2) of X cells, calculated using two different approaches, was found to be higher than that of Y cells (17,000 omega cm2). 6. The implication of these results for the integration of synaptic signals in the two classes of l.g.n. cells and the feasibility of differentiating between X and Y cells on the basis of their membrane properties are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Perkel D. H., Norris J. C., Peacock J. H. Electrotonic structure and specific membrane properties of mouse dorsal root ganglion neurons. J Neurophysiol. 1981 Jan;45(1):1–15. doi: 10.1152/jn.1981.45.1.1. [DOI] [PubMed] [Google Scholar]
- Bullier J., Norton T. T. X and Y relay cells in cat lateral geniculate nucleus: quantitative analysis of receptive-field properties and classification. J Neurophysiol. 1979 Jan;42(1 Pt 1):244–273. doi: 10.1152/jn.1979.42.1.244. [DOI] [PubMed] [Google Scholar]
- Burke W., Cole A. M. Extraretinal influences on the lateral geniculate nucleus. Rev Physiol Biochem Pharmacol. 1978;80:105–166. doi: 10.1007/3540084665_3. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. Blockade of amino acid-induced depolarizations and inhibition of excitatory post-synaptic potentials in rat dentate gyrus. J Physiol. 1983 Aug;341:627–640. doi: 10.1113/jphysiol.1983.sp014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. The ventral and dorsal lateral geniculate nucleus of the rat: intracellular recordings in vitro. J Physiol. 1987 Mar;384:587–601. doi: 10.1113/jphysiol.1987.sp016471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N., Parnavelas J. G. X- and Y-cells identified in the cat lateral geniculate nucleus in vitro. Brain Res. 1986 Aug 20;380(2):371–374. doi: 10.1016/0006-8993(86)90238-6. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966 Sep;128(1):21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Schaerer B., Vosmansky M. A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods. 1979 Dec;1(4):323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Hirsch J. C., Burnod Y., Korn H. Dorsolateral geniculate neurons in vitro: reduced postsynaptic excitability following repetitive activation of the optic tract. Neurosci Lett. 1985 Jul 4;58(1):151–156. doi: 10.1016/0304-3940(85)90345-3. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D. Passive cable properties of hippocampal CA3 pyramidal neurons. Cell Mol Neurobiol. 1981 Mar;1(1):41–55. doi: 10.1007/BF00736038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Understanding the intrinsic circuitry of the cat's lateral geniculate nucleus: electrical properties of the spine-triad arrangement. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):365–390. doi: 10.1098/rspb.1985.0067. [DOI] [PubMed] [Google Scholar]
- LeVay S., Ferster D. Relay cell classes in the lateral geniculate nucleus of the cat and the effects of visual deprivation. J Comp Neurol. 1977 Apr 15;172(4):563–584. doi: 10.1002/cne.901720402. [DOI] [PubMed] [Google Scholar]
- MORALES R., DUNCAN D., REHMET R. A DISTINCTIVE LAMINATED CYTOPLASMIC BODY IN THE LATERAL GENICULATE BODY NEURONS OF THE CAT. J Ultrastruct Res. 1964 Feb;10:116–123. doi: 10.1016/s0022-5320(64)90025-5. [DOI] [PubMed] [Google Scholar]
- Sato S., Tsukahara N. Some properties of the theoretical membrane transients in Rall's neuron model. J Theor Biol. 1976 Nov;63(1):151–163. doi: 10.1016/0022-5193(76)90090-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Hirsch H. V. A quantitative study of the occurrence and distribution of cytoplasmic laminated bodies in the lateral geniculate nucleus of the normal adult cat. J Comp Neurol. 1980 Jan 15;189(2):235–247. doi: 10.1002/cne.901890203. [DOI] [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res. 1986;63(1):1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Cable properties of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1984 Aug;52(2):278–289. doi: 10.1152/jn.1984.52.2.278. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Llinás R. Hippocampal pyramidal cells: significance of dendritic ionic conductances for neuronal function and epileptogenesis. J Neurophysiol. 1979 Mar;42(2):476–496. doi: 10.1152/jn.1979.42.2.476. [DOI] [PubMed] [Google Scholar]