Abstract
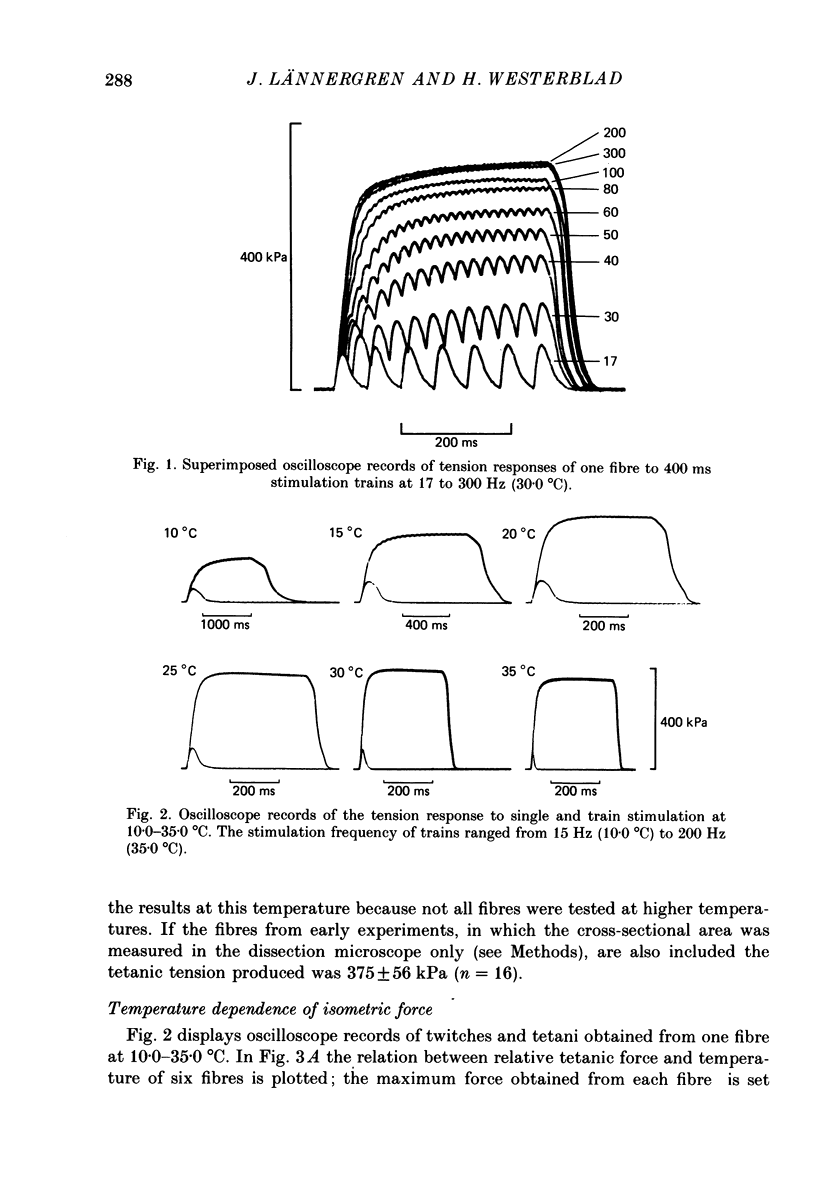
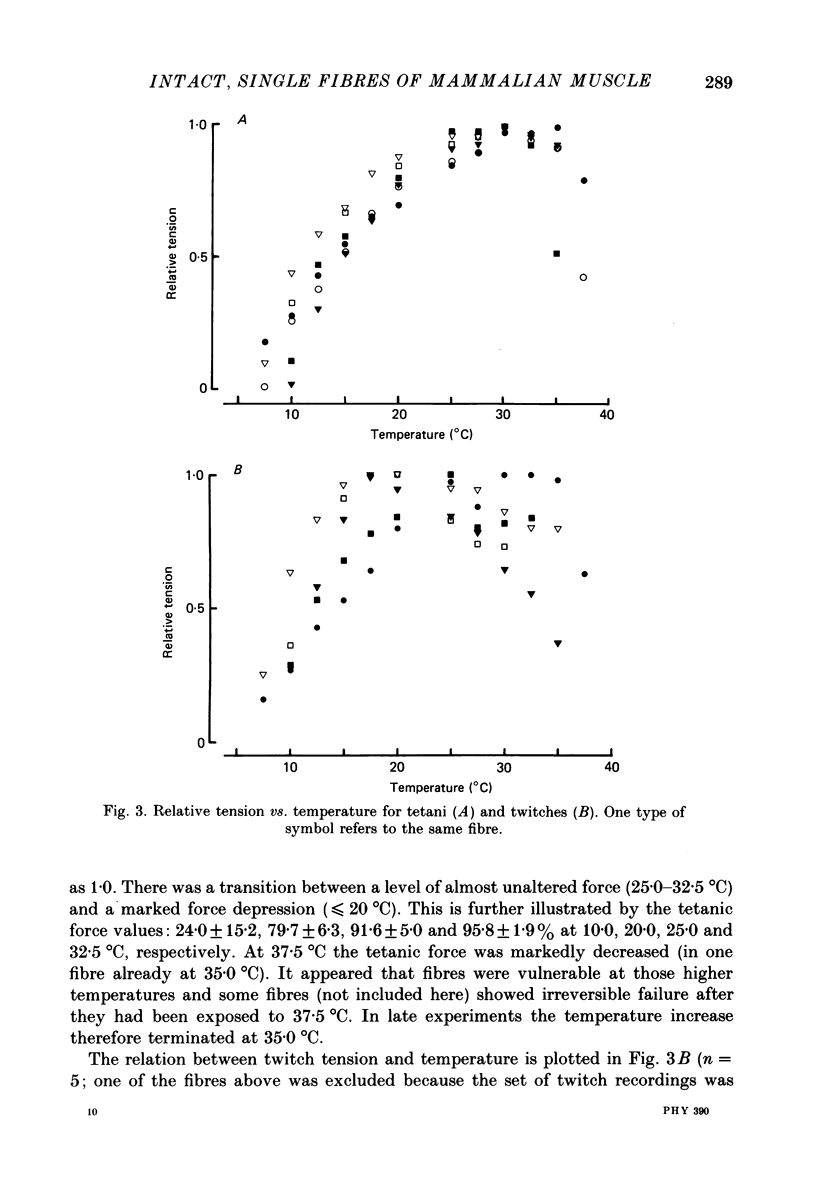
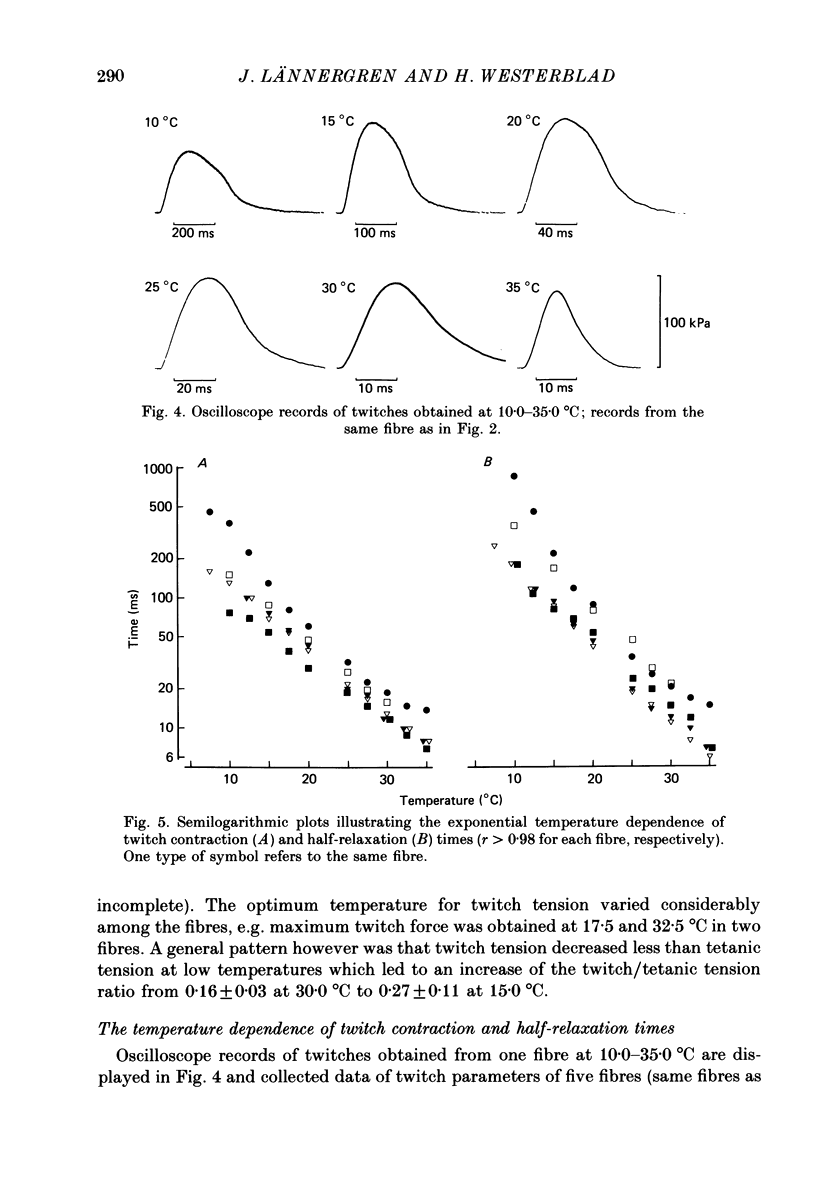
1. Isometric tension responses to electrical stimulation have been studied at 7.5 37.5 degrees C in single, intact fibres of the flexor digitorum brevis muscle of the mouse. A large number of reproducible tetani could be obtained at temperatures less than or equal to 35 degrees C. 2. The tetanic force per cross-sectional area generated at 25.0 degrees C was 375 +/- 56 kPa (mean +/- S.D., n = 16). 3. The curve relating maximum tetanic tension to temperature exhibited a transition between a level of almost unaltered force (25.0-32.5 degrees C) and a marked force decline (less than or equal to 20.0 degrees C). At temperatures higher than 35.0 degrees C force production was markedly depressed and this reduction was in some cases irreversible. 4. Twitch tension showed less regular dependence on temperature; it was reduced less than tetanic tension at low temperatures. Thus, the twitch/tetanus tension ratio was higher at low temperatures. 5. The times for twitch contraction and for twitch half-relaxation (i) ranged from 7 to 14 ms and from 6 to 15 ms at 35.0 degrees C and (ii) exhibited Q10 values of 3.2 +/- 0.4 and 4.0 +/- 0.6, respectively. 6. It is concluded that it is possible to use intact, single fibres dissected from mammalian skeletal muscle in physiological studies. Our results are close to previous results obtained from mammalian muscles except that the tetanic tension per cross-sectional area was found to be higher than commonly reported.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd I. A., Ward J. Motor control of nuclear bag and nuclear chain intrafusal fibres in isolated living muscle spindles from the cat. J Physiol. 1975 Jan;244(1):83–112. doi: 10.1113/jphysiol.1975.sp010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. E., Sichel F. J. THE MYOGRAM OF THE ISOLATED SKELETAL MUSCLE CELL. Science. 1930 Jul 4;72(1853):17–18. doi: 10.1126/science.72.1853.17. [DOI] [PubMed] [Google Scholar]
- Buller A. J., Kean C. J., Ranatunga K. W., Smith J. M. Temperature dependence of isometric contractions of cat fast and slow skeletal muscles. J Physiol. 1984 Oct;355:25–31. doi: 10.1113/jphysiol.1984.sp015403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968 Mar 23;217(5134):1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Gordon T., Stein R. B. Temperature effects on the kinetics of force generation in normal and dystrophic mouse muscles. Exp Neurol. 1985 Aug;89(2):348–360. doi: 10.1016/0014-4886(85)90095-0. [DOI] [PubMed] [Google Scholar]
- Hartree W., Hill A. V. The nature of the isometric twitch. J Physiol. 1921 Nov 18;55(5-6):389–411. doi: 10.1113/jphysiol.1921.sp001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G., Millman M., Eletr S. Temperature-induced transitions of function and structure in sarcoplasmic reticulum membranes. J Mol Biol. 1973 Dec 25;81(4):483–504. doi: 10.1016/0022-2836(73)90518-4. [DOI] [PubMed] [Google Scholar]
- Isaacson A., Hinkes M. J., Taylor S. R. Contracture and twitch potentiation of fast and slow muscles of the rat at 20 and 37 C. Am J Physiol. 1970 Jan;218(1):33–41. doi: 10.1152/ajplegacy.1970.218.1.33. [DOI] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of fiber bundles from the rat sternomastoid muscle. Exp Neurol. 1985 Sep;89(3):491–502. doi: 10.1016/0014-4886(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Ranatunga K. W. Influence of temperature on isometric tension development in mouse fast- and slow-twitch skeletal muscles. Exp Neurol. 1980 Nov;70(2):211–218. doi: 10.1016/0014-4886(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Ranatunga K. W., Wylie S. R. Temperature-dependent transitions in isometric contractions of rat muscle. J Physiol. 1983 Jun;339:87–95. doi: 10.1113/jphysiol.1983.sp014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton A. W. Isometric tension of glycerinated muscle fibers following adrenalectomy. Am J Physiol. 1967 Feb;212(2):313–316. doi: 10.1152/ajplegacy.1967.212.2.313. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Gordon T., Shriver J. Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J. 1982 Nov;40(2):97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs H. E., Luff A. R., Luff S. E. Force--sarcomere-length relation and filament length in rat extensor digitorum muscle. Adv Exp Med Biol. 1984;170:511–525. doi: 10.1007/978-1-4684-4703-3_44. [DOI] [PubMed] [Google Scholar]



