Abstract
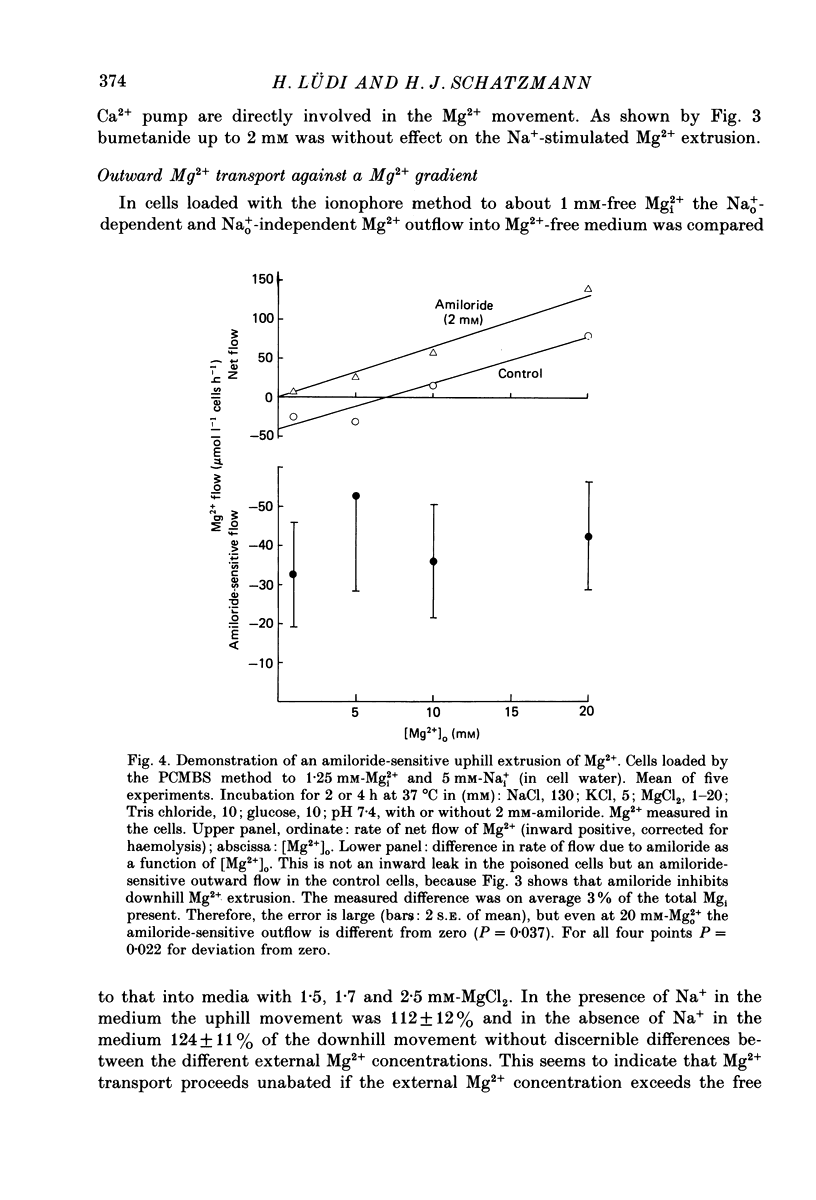
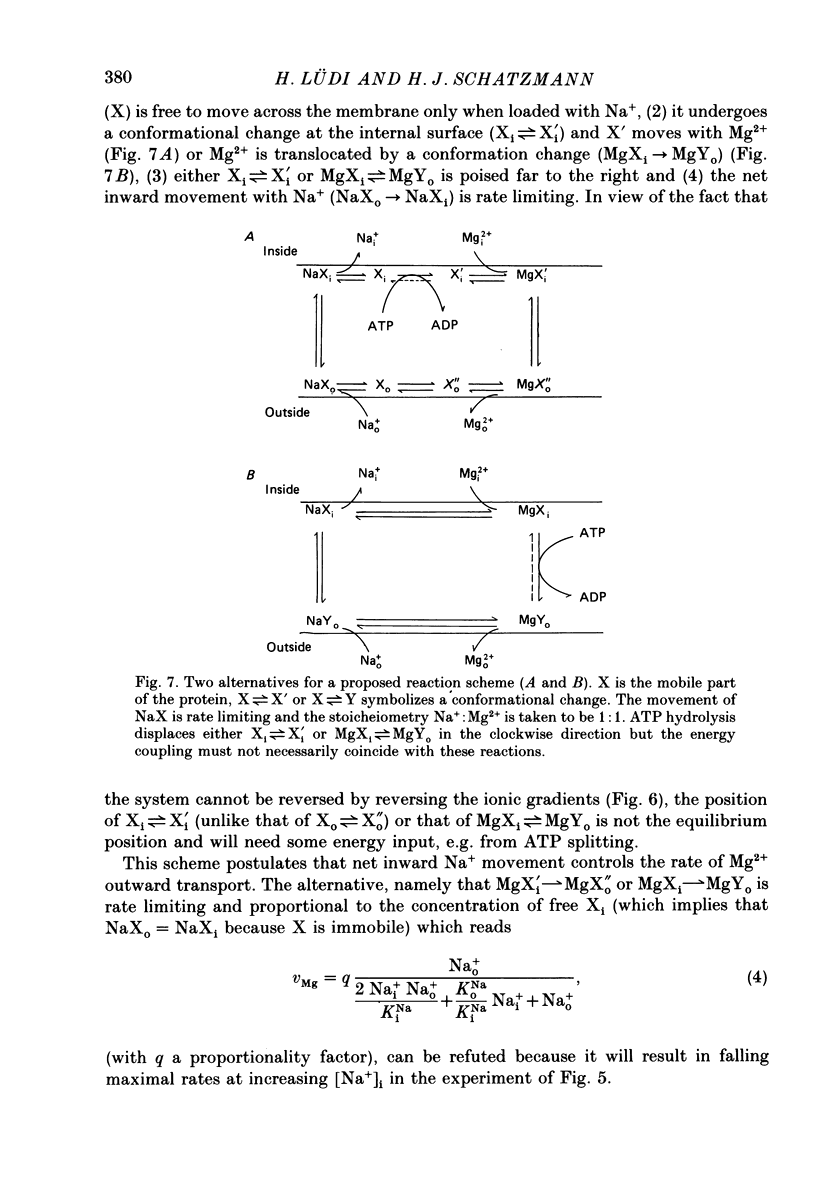
1. In agreement with the report by Féray & Garay (1986) it is shown that Mg2+ leaves human red cells mainly by a saturable pathway at a maximal rate of some 200 mumol 1(-1) cells h(-1) only if the medium contains Na+.Mg2+0.5 (i.e. the free Mg2+ concentration for half-maximal rate) at the internal membrane surface is 1.3 mM and the dissociation constant for Na+, KNa, at the external surface is 16-17 mM. Mg2+ shows co-operative behaviour. The Na+o-stimulated Mg2+ outflow is sensitive to millimolar amiloride concentrations. Implication of the Na+ or Ca+ pump can be ruled out. 2. Na+i is inhibitory by simple competition with Na+o. The affinity for Na+ inside is the same as outside. There is no detectable competition between Na+i and Mg2+i. 3. At approximately 1 mM-[Mg2+]i the outward Mg2+ movement stimulated by Na+o still proceeds when [Mg2+]o is increased up to 20 mM. Thus the Mg2+ movement is uphill and the apparent Mg2+0.5 at the external surface is larger than 20 mM. 4. Reversing the Na+ gradient (making [Na+]i greater than [Na+]o) does not elicit an inward Mg2+ movement, even if [Mg2+]o is simultaneously made larger than [Mg2+]i. 5. The Na+o-dependent Mg2+ outflow ceases nearly completely (falling to 5% of the control) in metabolically depleted cells. 6. The behaviour observed is compatible with the assumptions that (1) the system possesses distinct binding sites for Na+ and for Mg2+, (2) the ionophoric moiety is passively mobile when loaded with Na+, (3) the movement of the Na+ form is rate limiting, and (4) the Mg2+ form preferentially moves in the outward direction owing to an input of metabolic energy (ATP hydrolysis) and is immobile in starved cells. 7. Mg2+ may be required at a further site(s) not involved in the actual Mg2+ translocation but in the energy input. The simple kinetics suggesting translocation of one Na+ ion in exchange for one Mg2+ ion were found in selected cells of average maximum transport rate (Vmax) and may not hold for all cell specimens. 8. The conclusion is that the system is a Mg2+ extrusion pump driven by metabolic energy directly and not by the inward Na+ gradient, although net inward Na+ movement is necessary to bring the ionophoric part of the system back to the in-position. It appears that in intact cells the system operates far below saturation by Mg2+i and, by compensating for an inward leak of less than 5 mumol,1(-1) cells h(-1), sets the internal free Mg2+ concentration at about 0.5 of the equilibrium value.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. Studies on the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):286–294. doi: 10.1042/bj0320286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., McGuigan J. A. Free intracellular magnesium concentration in ferret ventricular muscle measured with ion selective micro-electrodes. Q J Exp Physiol. 1986 Jul;71(3):467–473. doi: 10.1113/expphysiol.1986.sp003005. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Lew V. L. Magnesium buffering in intact human red blood cells measured using the ionophore A23187. J Physiol. 1980 Aug;305:13–30. doi: 10.1113/jphysiol.1980.sp013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Flatman P., Lew V. L. Use of ionophore A23187 to measure and to control free and bound cytoplasmic Mg in intact red cells. Nature. 1977 May 26;267(5609):360–362. doi: 10.1038/267360a0. [DOI] [PubMed] [Google Scholar]
- Féray J. C., Garay R. An Na+-stimulated Mg2+-transport system in human red blood cells. Biochim Biophys Acta. 1986 Mar 27;856(1):76–84. doi: 10.1016/0005-2736(86)90012-x. [DOI] [PubMed] [Google Scholar]
- GINSBURG S., SMITH J. G., GINSBURG F. M., REARDON J. Z., AIKAWA J. K. Magnesium metabolism of human and rabbit erythrocytes. Blood. 1962 Dec;20:722–729. [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Cation loading of red blood cells. J Physiol. 1967 Nov;193(2):459–466. doi: 10.1113/jphysiol.1967.sp008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Moore R. D. 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem. 1980 May 10;255(9):3987–3993. [PubMed] [Google Scholar]
- Günther T., Vormann J. Mg2+ efflux is accomplished by an amiloride-sensitive Na+/Mg2+ antiport. Biochem Biophys Res Commun. 1985 Jul 31;130(2):540–545. doi: 10.1016/0006-291x(85)90450-4. [DOI] [PubMed] [Google Scholar]
- Güther T., Vormann J., Förster R. Regulation of intracellular magnesium by Mg2+ efflux. Biochem Biophys Res Commun. 1984 Feb 29;119(1):124–131. doi: 10.1016/0006-291x(84)91627-9. [DOI] [PubMed] [Google Scholar]
- Lloyd S., Pickford M. The effect of oxytocin and adrenaline on blood flow in the hind limb of the dog following chronic lumbar sympathectomy. J Physiol. 1967 Sep;192(1):43–52. doi: 10.1113/jphysiol.1967.sp008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. A mechanism for Na/Ca transport. J Gen Physiol. 1977 Dec;70(6):681–695. doi: 10.1085/jgp.70.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. The use of ionophores of rapid loading of human red cells with radioactive cations for cation-pump studies. J Membr Biol. 1976 May;26(4):357–370. doi: 10.1007/BF01868883. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M. Magnesium metabolism. Ergeb Physiol. 1967;59:185–296. doi: 10.1007/BF02269144. [DOI] [PubMed] [Google Scholar]


