Abstract
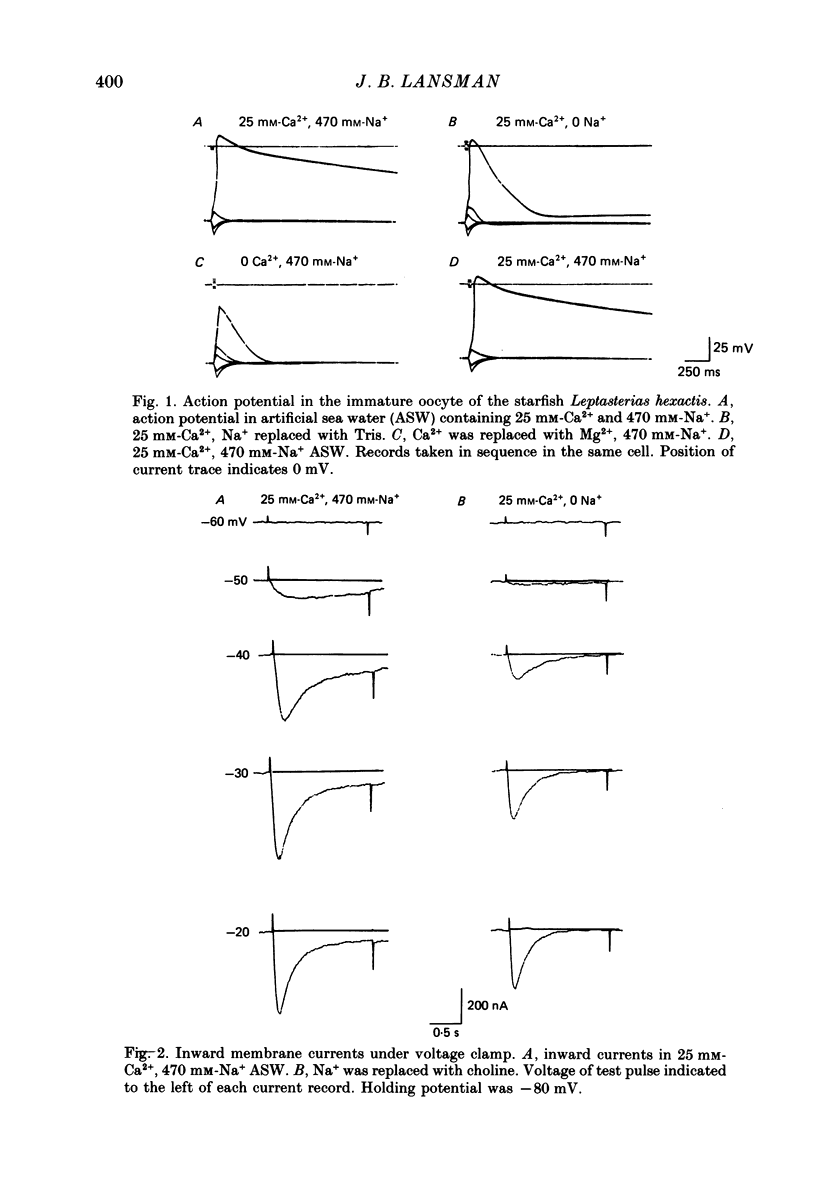
1. Inward currents in the immature oocyte of the starfish Leptasterias hexactis were studied with a two-micro-electrode voltage clamp. Experiments investigated the role of Ca2+ in the Na+-dependent plateau of the action potential. 2. Voltage steps more positive than -55 mV produced inward currents in normal sea water that activated and then decayed to a non-zero level with a double-exponential time course. Returning the voltage to the resting potential produced an inward tail current that relaxed slowly to zero with a time course of seconds. 3. Replacing Na+ with choline abolished the slowly decaying component as well as the slow tail current which followed the end of the voltage pulse. This suggested that inward current in Na+-containing sea water consisted of a rapidly decaying component that flowed through Ca2+ channels and a more slowly decaying component carried by Na+. 4. Ca2+ current was isolated in Na+-free sea water. Activation followed a sigmoidal time course that could be described with m2 kinetics. Inactivation during a maintained depolarization followed first-order kinetics and was voltage dependent. 5. When Ba2+ was substituted for Ca2+ as the divalent ion charge carrier, inward currents in Na+-containing sea water decayed along a single-exponential time course. The absence of a slowly decaying Na+ current in Ba2+-containing sea water suggested that Na+ current depended on Ca2+ influx. 6. The effects of altering Ca2+ influx on the time course of Na+ current were investigated. Na+ current decayed more rapidly as the test pulse potential was made more positive, while raising [Ca2+]o slowed the decaying phase without altering its dependence on membrane potential. 7. Tail currents measured after rapidly stepping the membrane potential back to the resting level consisted of a fast component associated with the closing of Ca2+ channels and a slow component that was abolished by removing Na+. 8. The variation of the amplitude of the slow component of tail current with the duration of the voltage-clamp pulse indicated that Na+ current is associated with a time-dependent component of membrane conductance. 9. Possible mechanisms for the slowly decaying Na+ current are considered. The results are discussed in relation to the idea that the conductance change to Na+ follows the time course of Ca2+ accumulation and removal from the cytoplasm.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976 May 6;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B. Voltage-clamp study of the conductance activated at fertilization in the starfish egg. J Physiol. 1983 Dec;345:353–372. doi: 10.1113/jphysiol.1983.sp014982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Action potential and non-linear current-voltage relation in starfish oocytes. J Physiol. 1975 Mar;246(1):37–54. doi: 10.1113/jphysiol.1975.sp010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. Fast polyspermy block and activation potential. Electrophysiological bases for their changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):341–354. doi: 10.1016/0012-1606(79)90032-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Hirai S. Fast polyspermy block and activation potential. Correlated changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):327–340. doi: 10.1016/0012-1606(79)90031-9. [DOI] [PubMed] [Google Scholar]
- Moody W. J., Lansman J. B. Developmental regulation of Ca2+ and K+ currents during hormone-induced maturation of starfish oocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):3096–3100. doi: 10.1073/pnas.80.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Steinhardt R. A. An electrophysiological study of the membrane properties of the immature and mature oocyte of the batstar, Patiria miniata. Dev Biol. 1976 Jan;48(1):148–162. doi: 10.1016/0012-1606(76)90053-1. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Permeation of divalent and monovalent cations through the ovarian oocyte membrane of the mouse. J Physiol. 1983 Jun;339:631–642. doi: 10.1113/jphysiol.1983.sp014739. [DOI] [PMC free article] [PubMed] [Google Scholar]



