Abstract
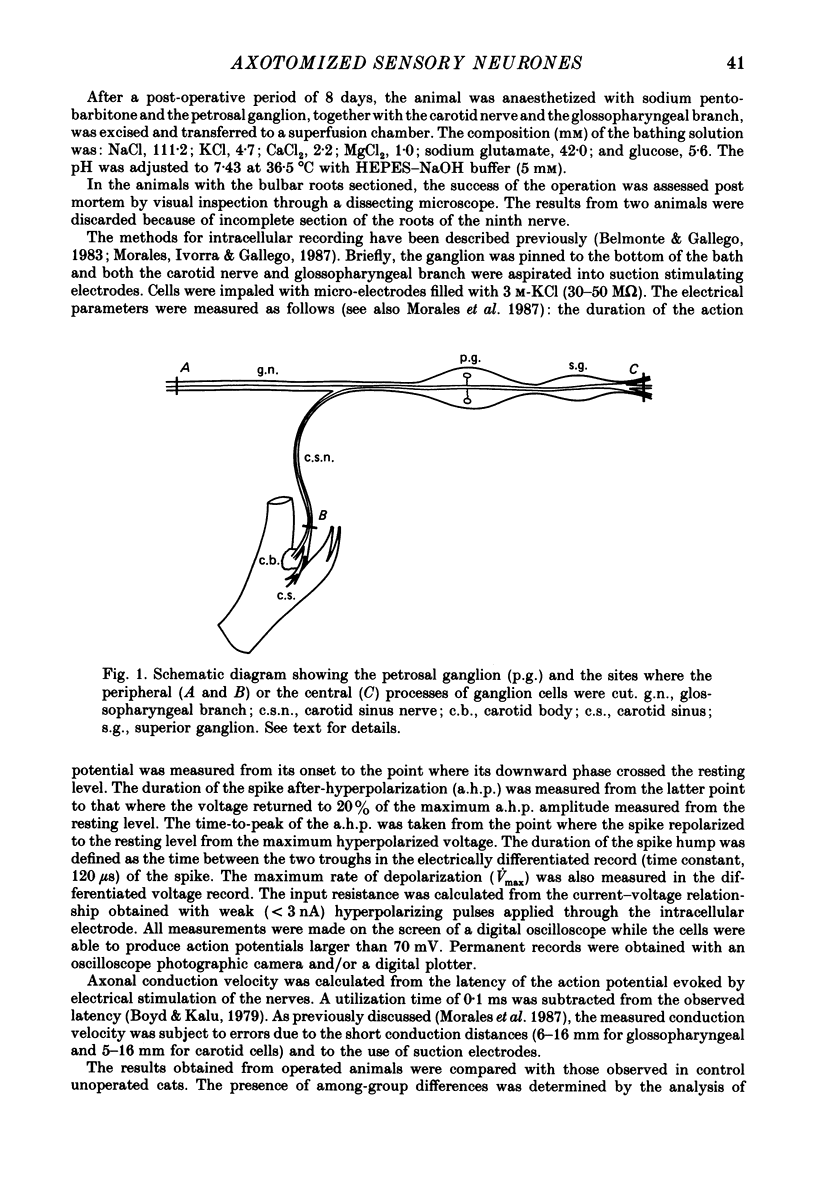
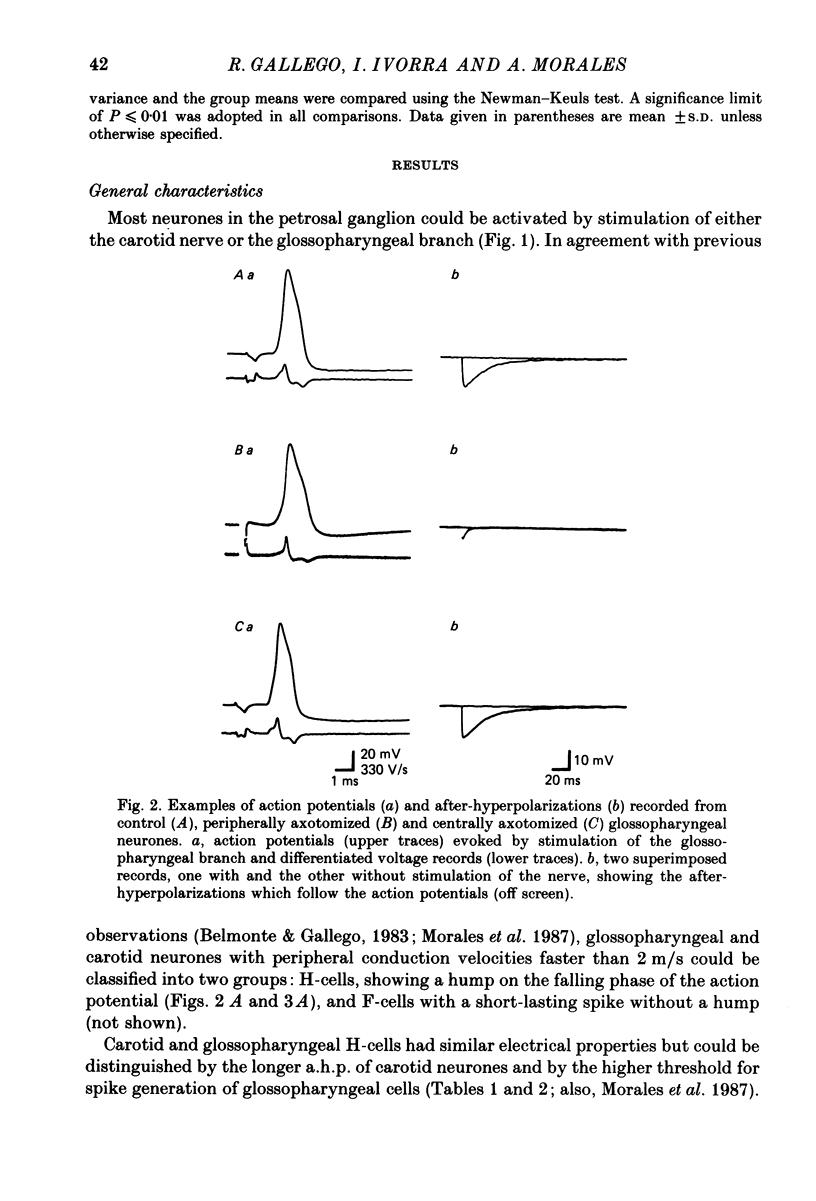
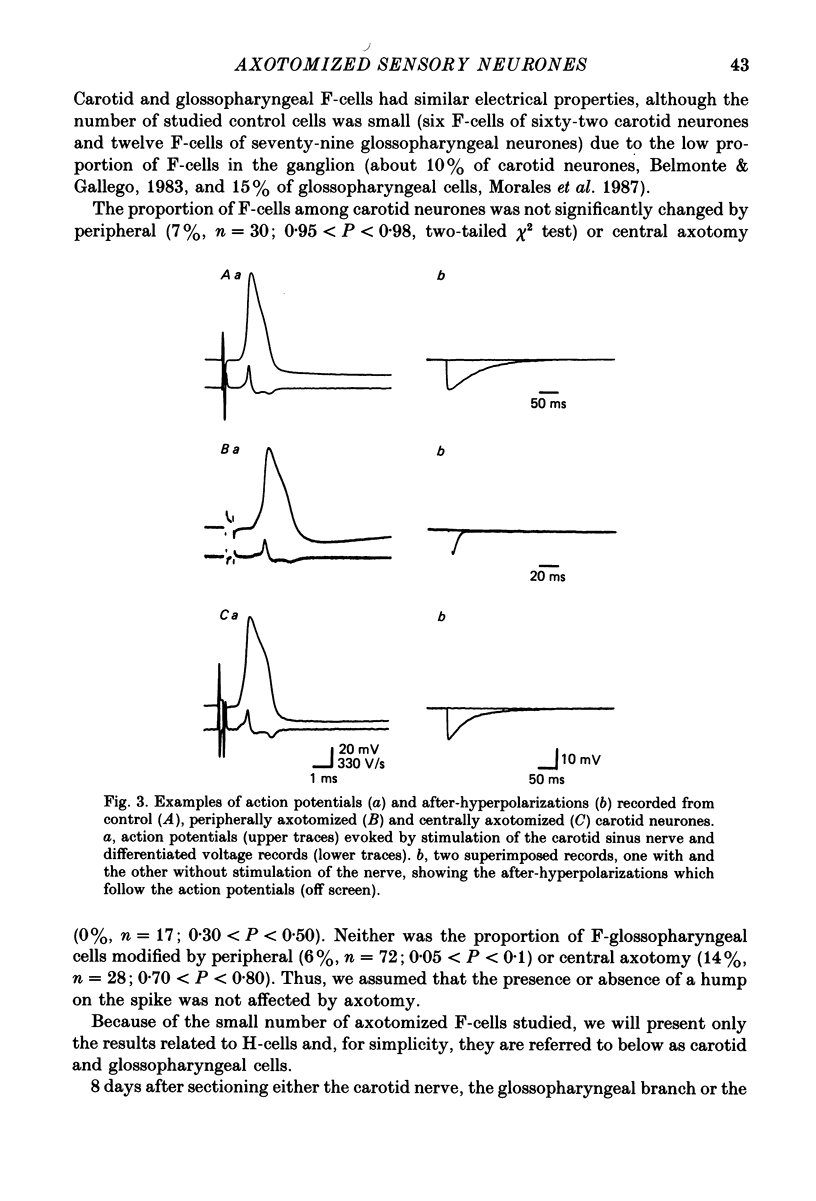
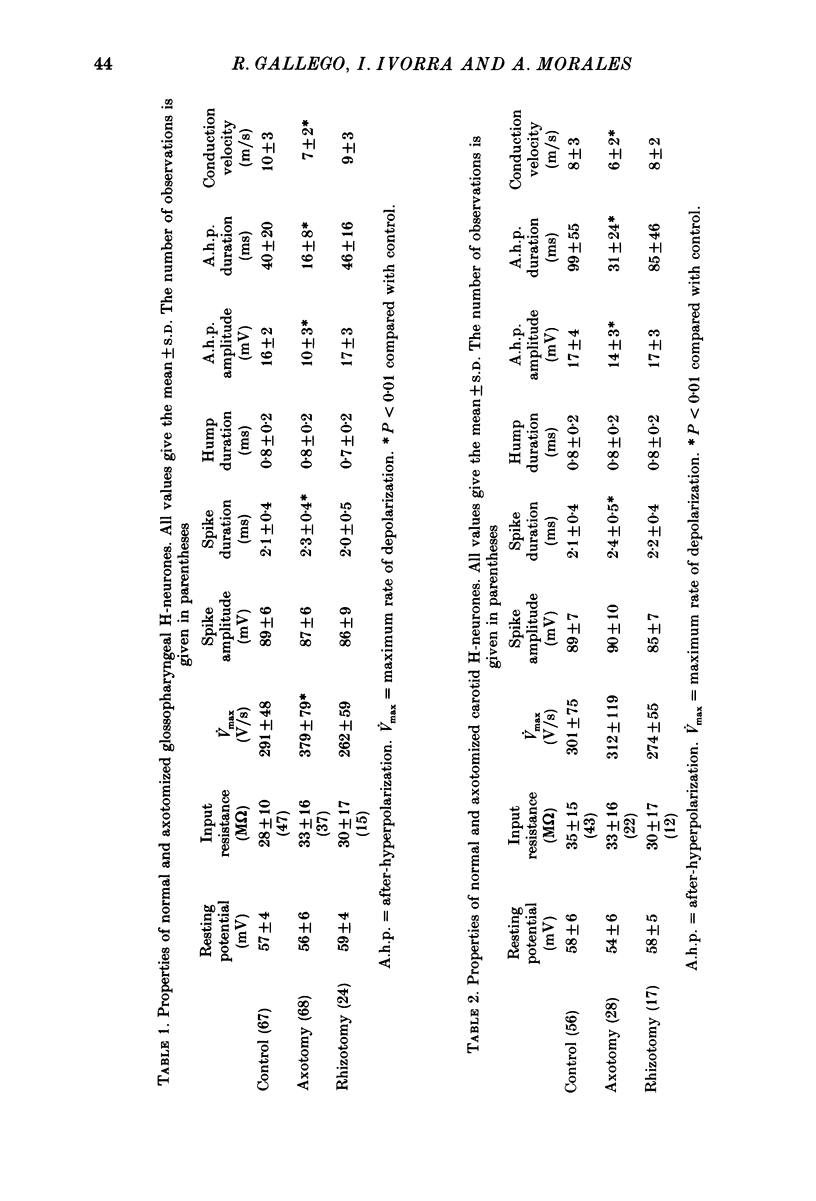
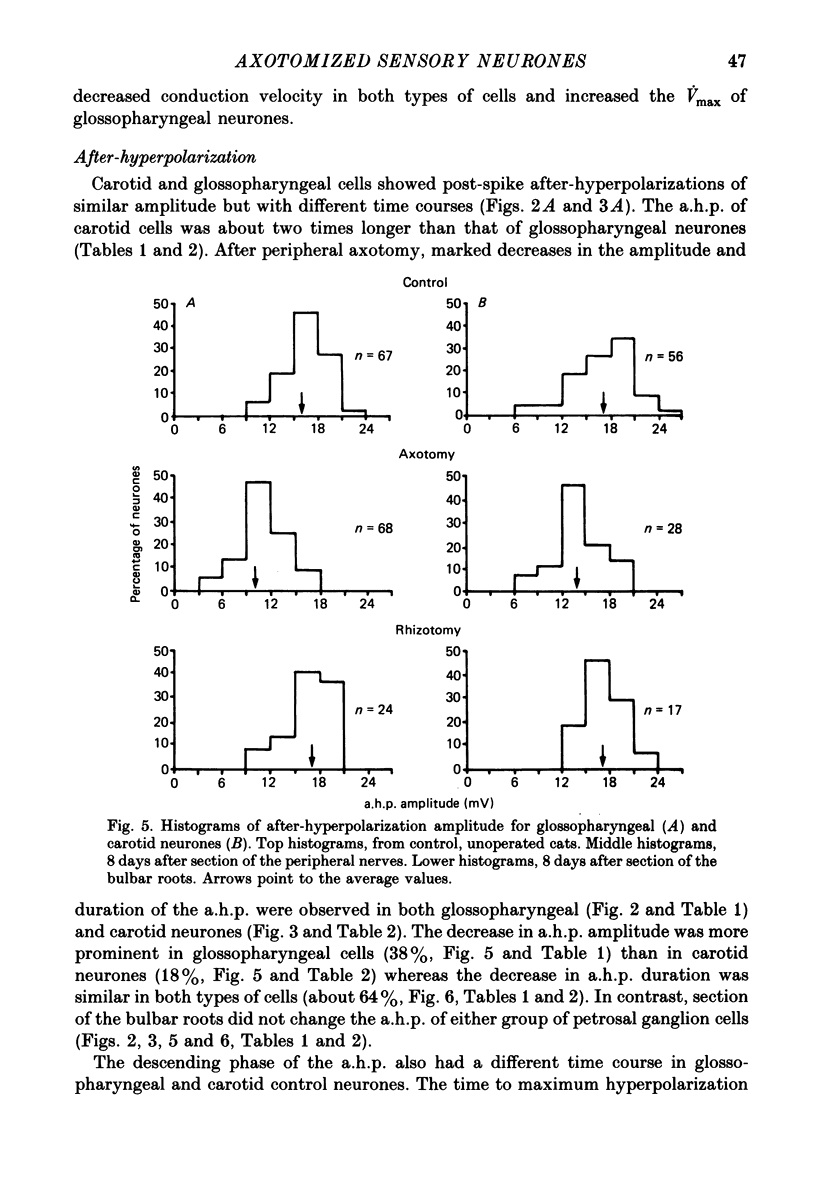
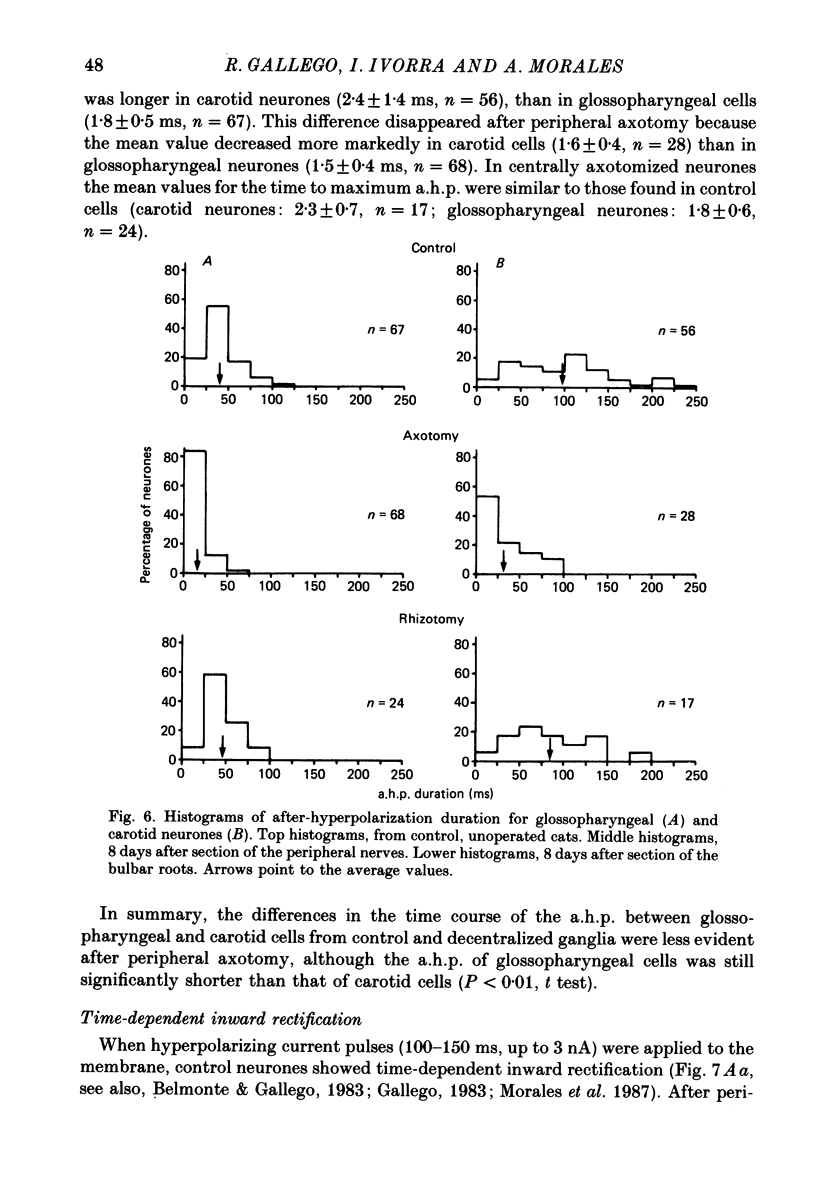
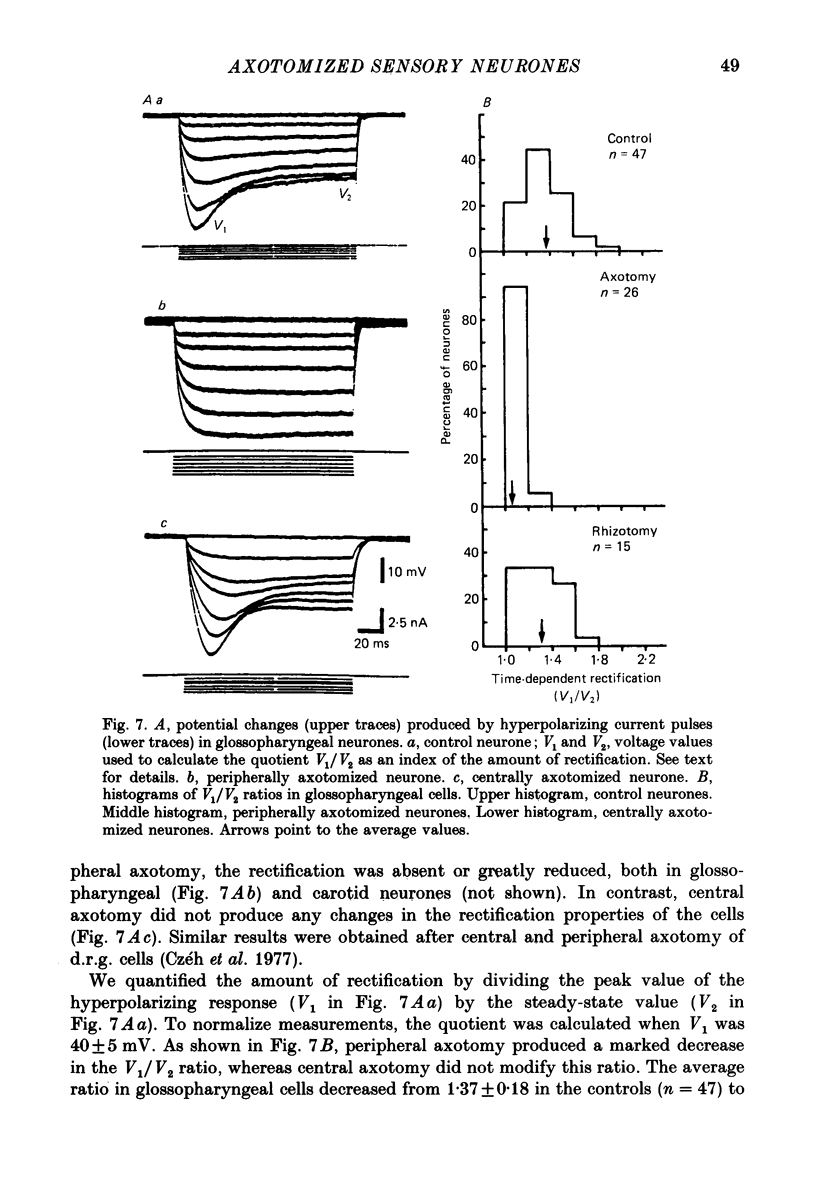
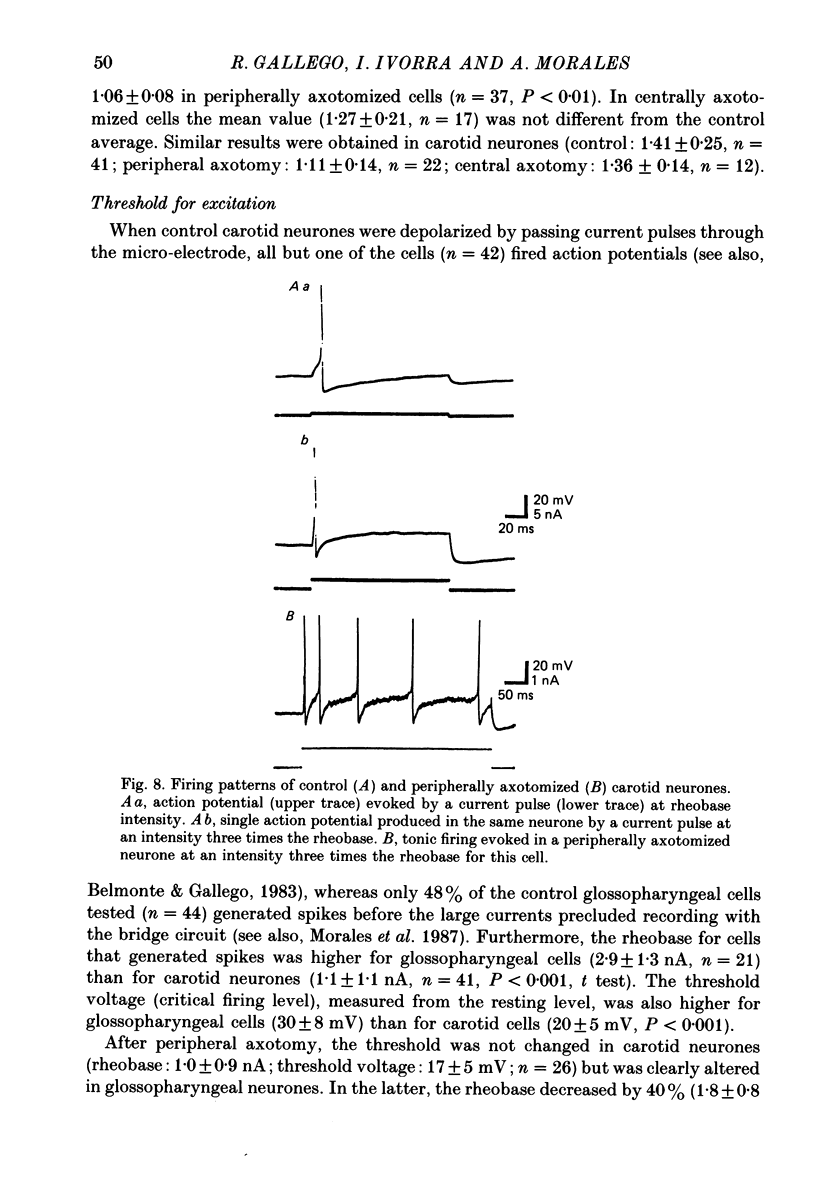
1. The properties of sensory neurones in the petrosal ganglion of the cat were examined in vitro with intracellular electrodes 8 days after section of the central (bulbar roots) or peripheral process. Two types of cells, both with conduction velocities faster than 2 m/s and with humps on the falling phases of their action potentials (H-neurones), were studied: glossopharyngeal neurones arising from the tongue and pharynx, and carotid neurones originating in the carotid body and carotid sinus. 2. Peripheral axotomy produced an increase in action potential duration and a marked decrease in the amplitude and duration of the spike after-hyperpolarization in both glossopharyngeal and carotid neurones. 3. The maximum rate of depolarization of the action potential increased after peripheral axotomy in glossopharyngeal cells but did not change in carotid neurones. 4. The time-dependent inward rectification in response to hyperpolarizing pulses was markedly reduced in both types of cells after peripheral axotomy. 5. Section of the peripheral process produced a decrease of the rheobase of glossopharyngeal cells, but not of carotid neurones. After axotomy the proportion of cells giving tonic discharges in response to long depolarizing pulses increased from 13 to 54% among carotid neurones but did not change in glossopharyngeal cells. 6. No significant changes in membrane potential or input resistance of either group of cells were found after peripheral axotomy. 7. Central axotomy did not produce any changes in the electrophysiological properties of glossopharyngeal or carotid neurones. 8. Peripheral conduction velocity was decreased in both types of cells after peripheral axotomy, but did not change after section of the bulbar roots. 9. It is concluded that the electrical properties of sensory neurones are modified after peripheral axotomy but not after central axotomy. Furthermore, the changes produced by peripheral axotomy are different in neurones innervating different peripheral targets. 10. The possibility that some electrical properties of sensory neurones are maintained by their peripheral targets is discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Galvan M. Voltage-dependent currents of vertebrate neurons and their role in membrane excitability. Adv Neurol. 1986;44:137–170. [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C., Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. J Physiol. 1983 Sep;342:603–614. doi: 10.1113/jphysiol.1983.sp014871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci Lett. 1984 Oct 12;51(2):241–246. doi: 10.1016/0304-3940(84)90558-5. [DOI] [PubMed] [Google Scholar]
- Boyd I. A., Kalu K. U. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol. 1979 Apr;289:277–297. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csillik B., Schwab M. E., Thoenen H. Transganglionic regulation of central terminals of dorsal root ganglion cells by nerve growth factor (NGF). Brain Res. 1985 Apr 1;331(1):11–15. doi: 10.1016/0006-8993(85)90709-7. [DOI] [PubMed] [Google Scholar]
- Czéh G., Gallego R., Kudo N., Kuno M. Evidence for the maintenance of motoneurone properties by muscle activity. J Physiol. 1978 Aug;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh G., Kudo N., Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol. 1977 Aug;270(1):165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M., Govrin-Lippmann R. Selective regeneration of sensory fibers following nerve crush injury. Exp Neurol. 1979 Aug;65(2):243–254. doi: 10.1016/0014-4886(79)90094-3. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K., MILEDI R. Delayed effects of peripheral severance of afferent nerve fibres on the efficacy of their central synapses. J Physiol. 1959 Jan 28;145(1):204–220. doi: 10.1113/jphysiol.1959.sp006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farel P. B. Reflex activity of regenerating frog spinal motoneurons. Brain Res. 1978 Dec 15;158(2):331–341. doi: 10.1016/0006-8993(78)90679-0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Wall P. D., Goedert M., Emson P. C. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985 Apr 15;332(1):131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Gallego R., Kuno M., Núez R., Snider W. D. Dependence of motoneurone properties on the length of immobilized muscle. J Physiol. 1979 Jun;291:179–189. doi: 10.1113/jphysiol.1979.sp012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J Physiol. 1983 Sep;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Takahashi T. The calcium component of the action potential in spinal motoneurones of the rat. J Physiol. 1983 Feb;335:89–100. doi: 10.1113/jphysiol.1983.sp014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO M. The electrical activity of spinal ganglion cells investigated with intracellular microelectrodes. Jpn J Physiol. 1957 Dec 20;7(4):297–323. doi: 10.2170/jjphysiol.7.297. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G., Weight F. F. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol. 1986 Mar;55(3):527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Yip H. K. Central nervous system and peripheral nerve growth factor provide trophic support critical to mature sensory neuronal survival. 1985 Apr 25-May 1Nature. 314(6013):751–752. doi: 10.1038/314751a0. [DOI] [PubMed] [Google Scholar]
- Kelly M. E., Gordon T., Shapiro J., Smith P. A. Axotomy affects calcium-sensitive potassium conductance in sympathetic neurones. Neurosci Lett. 1986 Jun 18;67(2):163–168. doi: 10.1016/0304-3940(86)90391-5. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. EGTA and motoneuronal after-potentials. J Physiol. 1978 Feb;275:199–223. doi: 10.1113/jphysiol.1978.sp012186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Barde Y. A., Davies A. M., Rohrer H. Differences and similarities in the neurotrophic growth factor requirements of sensory neurons derived from neural crest and neural placode. J Cell Sci Suppl. 1985;3:115–129. doi: 10.1242/jcs.1985.supplement_3.12. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., James M. H., Russell R. J., Kelly J. S., Pasternak C. A. Changes in excitability induced by herpes simplex viruses in rat dorsal root ganglion neurons. J Neurosci. 1986 Feb;6(2):391–402. doi: 10.1523/JNEUROSCI.06-02-00391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. Selective block of inward but not outward rectification in rat sensory neurones infected with herpes simplex virus. J Physiol. 1986 Jun;375:327–338. doi: 10.1113/jphysiol.1986.sp016119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McINTYRE A. K., BRADLEY K., BROCK L. G. Responses of motoneurons undergoing chromatolysis. J Gen Physiol. 1959 May 20;42(5):931–958. doi: 10.1085/jgp.42.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A., Ivorra I., Gallego R. Membrane properties of glossopharyngeal sensory neurons in the petrosal ganglion of the cat. Brain Res. 1987 Jan 20;401(2):340–346. doi: 10.1016/0006-8993(87)91418-1. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich K. M., Yip H. K., Osborne P. A., Schmidt R. E., Johnson E. M., Jr Role of nerve growth factor in the adult dorsal root ganglia neuron and its response to injury. J Comp Neurol. 1984 Nov 20;230(1):110–118. doi: 10.1002/cne.902300110. [DOI] [PubMed] [Google Scholar]
- Rose R. D., Koerber H. R., Sedivec M. J., Mendell L. M. Somal action potential duration differs in identified primary afferents. Neurosci Lett. 1986 Jan 30;63(3):259–264. doi: 10.1016/0304-3940(86)90366-6. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Pearson J., Johnson E. M. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res. 1982 Jul 29;244(2):378–381. doi: 10.1016/0006-8993(82)90102-0. [DOI] [PubMed] [Google Scholar]
- Titmus M. J., Faber D. S. Altered excitability of goldfish Mauthner cell following axotomy. II. Localization and ionic basis. J Neurophysiol. 1986 Jun;55(6):1440–1454. doi: 10.1152/jn.1986.55.6.1440. [DOI] [PubMed] [Google Scholar]



