Abstract
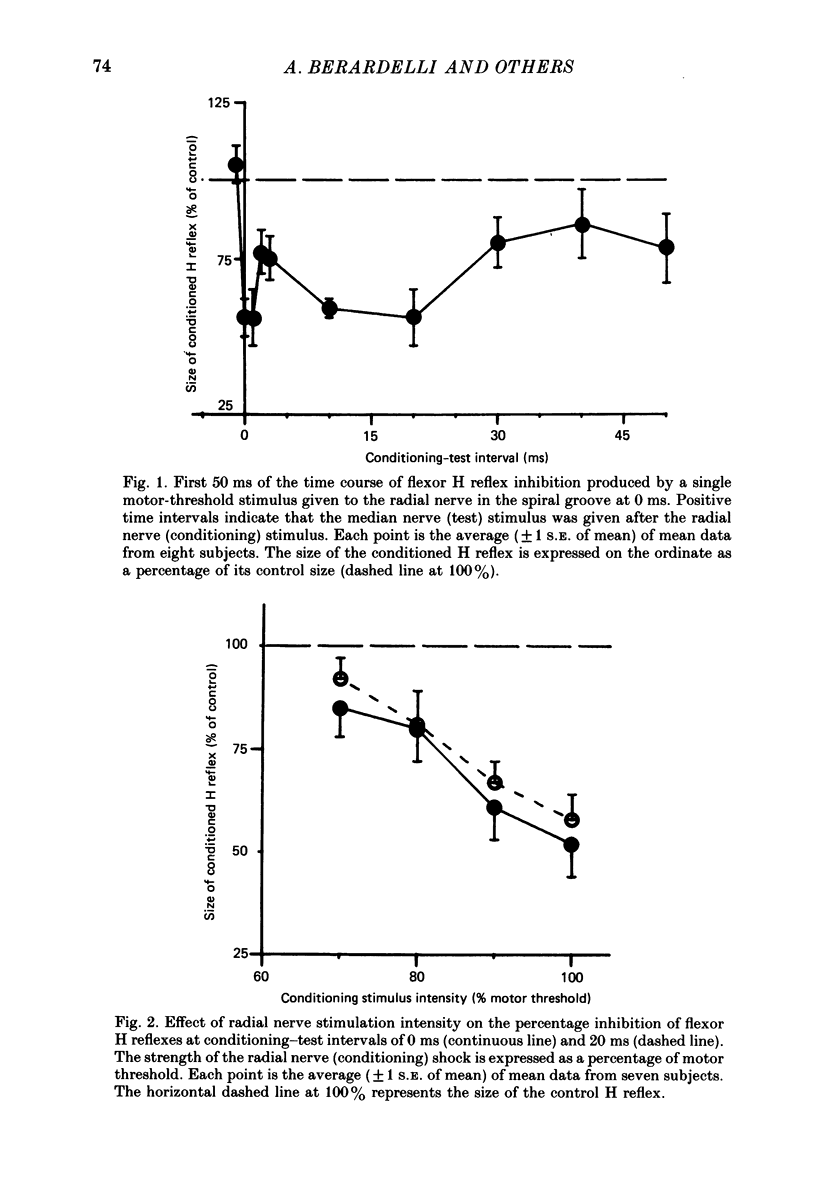
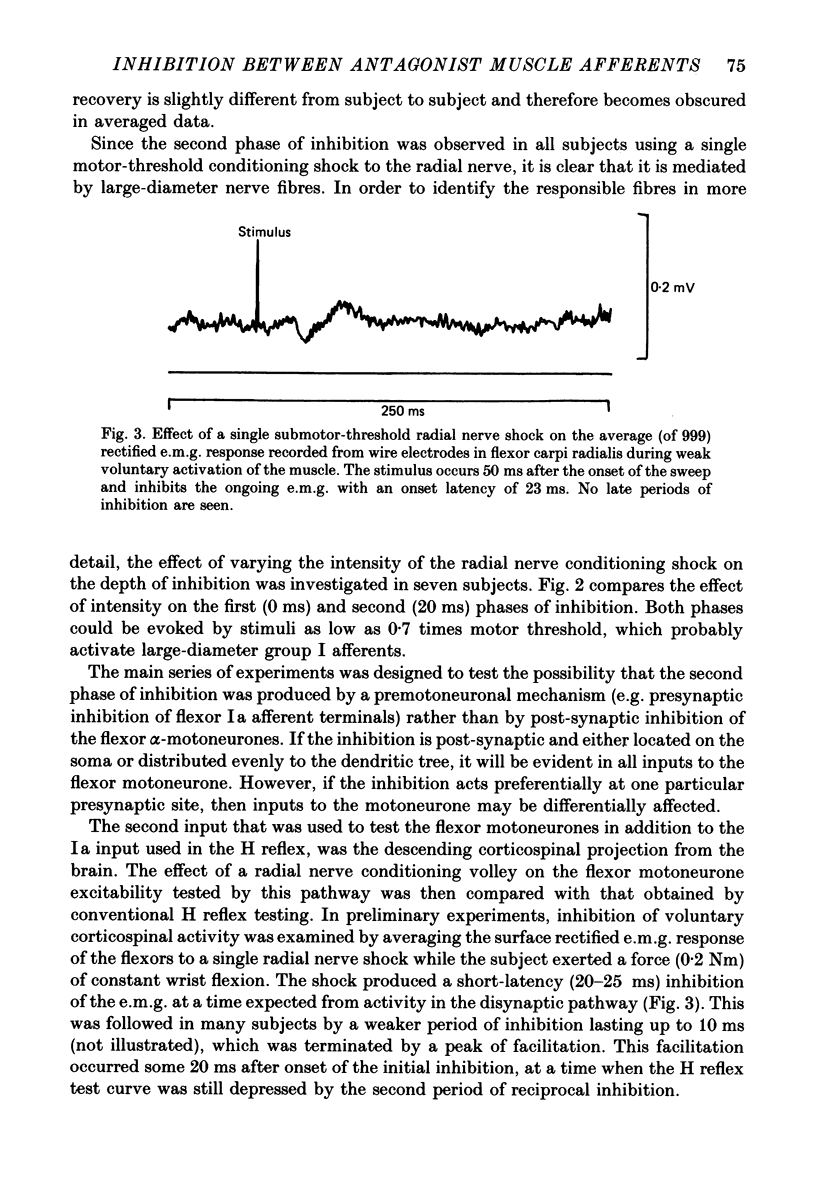
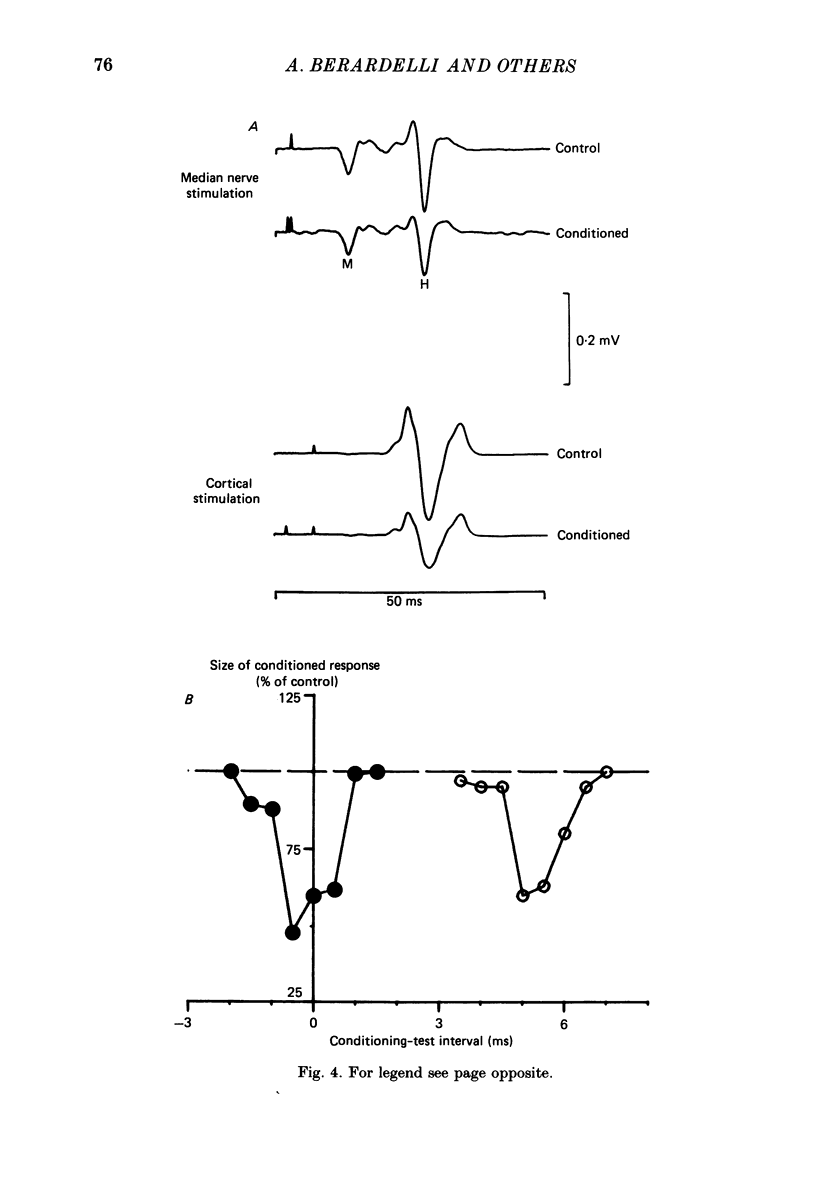
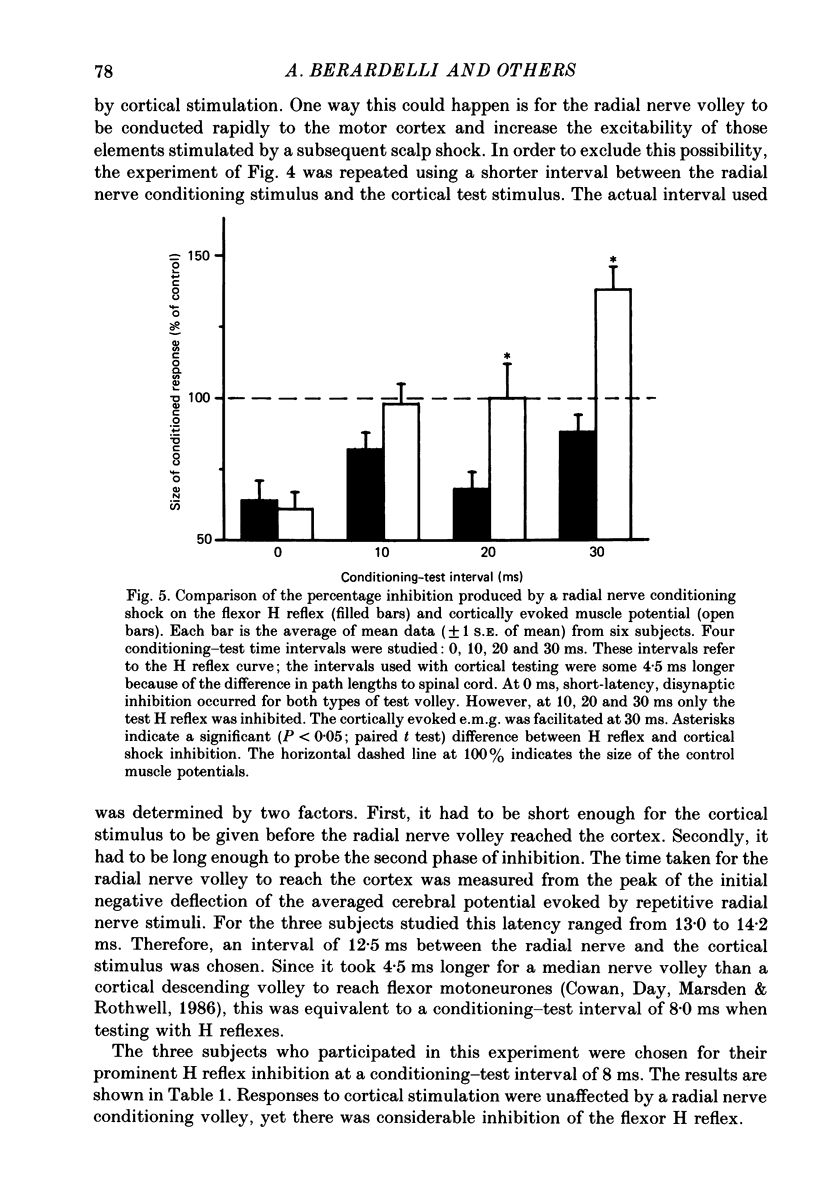
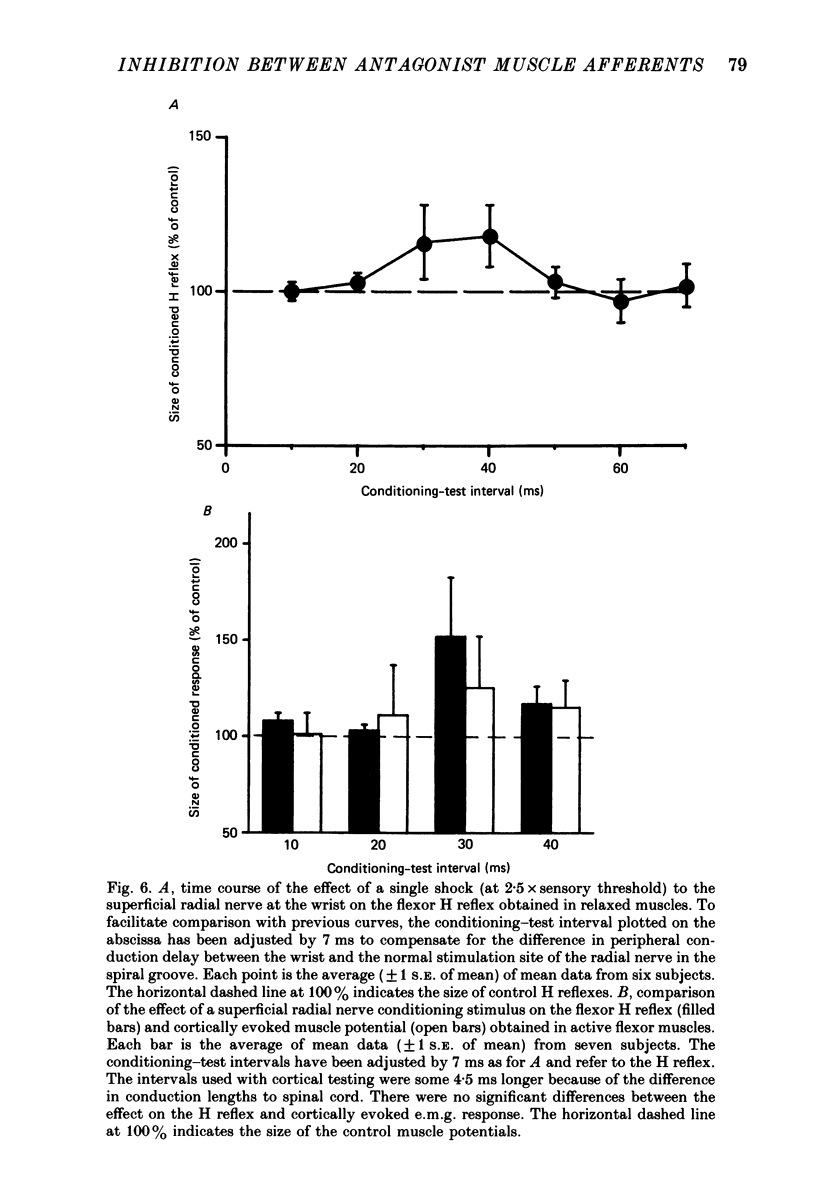
1. A single motor-threshold electrical stimulus to the radial nerve in the spiral groove produced three phases of inhibition in the H reflex of relaxed wrist and finger flexor muscles. The first phase of inhibition occurred with a conditioning-test interval of -1 to 3 ms and represents disynaptic Ia reciprocal inhibition of flexor motoneurones by afferent input from extensor muscles. The second phase occurred with a conditioning-test interval of 5-40 ms. The characteristics of the second inhibitory phase have been investigated in the present study. 2. The threshold intensity of radial nerve stimulation required to produce the second phase of inhibition was 0.7 X motor threshold and was the same as that required to produce the first (disynaptic) phase of inhibition. 3. The time course of the effect of a radial nerve stimulus on the size of e.m.g. response to transcranial electrical stimulation of the motor cortex also was studied. The results were compared to those obtained with H reflex testing. The time course and depth of the first phase of inhibition were identical with both test inputs. The second phase of inhibition, however, was seen only with the H reflex test stimulus. When the corticospinal volley was timed to arrive at the cervical cord 30 ms after the radial nerve afferent volley, the flexor muscle response was facilitated rather than inhibited. 4. A single stimulus (2-3 X sensory threshold) to the superficial (cutaneous) branch of the radial nerve at the wrist failed to produce inhibition of the flexor H reflex. Instead, the H reflex was facilitated when the median nerve Ia volley (for the H reflex) was timed to arrive at the cervical cord between 30-40 ms after the radial nerve cutaneous afferent volley. In contrast to results using mixed nerve stimulation in the spiral groove, there was no difference between the effect of this cutaneous input on the cortically evoked e.m.g. response and the H reflex. 5. We conclude that the second phase of flexor H reflex inhibition is produced by activity in large-diameter (group I) extensor muscle afferents which acts on the H reflex pathway at a premotoneuronal site.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth B., Grimby L., Kugelberg E. Comparison of voluntary and reflex activation of motor units. Functional organization of motor neurones. J Neurol Neurosurg Psychiatry. 1967 Apr;30(2):91–98. doi: 10.1136/jnnp.30.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A., Rothwell J. C., Day B. L., Kachi T., Marsden C. D. Duration of the first agonist EMG burst in ballistic arm movements. Brain Res. 1984 Jun 18;304(1):183–187. doi: 10.1016/0006-8993(84)90879-5. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol. 1983 Jun;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia M. R., McComas A. J., Upton A. R., Blogg T. Cutaneous reflexes in small muscles of the hand. J Neurol Neurosurg Psychiatry. 1973 Dec;36(6):960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J. M., Day B. L., Marsden C., Rothwell J. C. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol. 1986 Aug;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON G. D. The relative excitability and conduction velocity of sensory and motor nerve fibres in man. J Physiol. 1956 Feb 28;131(2):436–451. doi: 10.1113/jphysiol.1956.sp005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Marsden C. D., Obeso J. A., Rothwell J. C. Reciprocal inhibition between the muscles of the human forearm. J Physiol. 1984 Apr;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tohamy A., Sedgwick E. M. Spinal inhibition in man: depression of the soleus H reflex by stimulation of the nerve to the antagonist muscle. J Physiol. 1983 Apr;337:497–508. doi: 10.1113/jphysiol.1983.sp014638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Meunier S., Pierrot-Deseilligny E., Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986 Aug;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Hultborn H., Larsson R., Lundberg A. Reciprocal inhibition during the tonic stretch reflex in the decerebrate cat. J Physiol. 1978 Nov;284:345–369. doi: 10.1113/jphysiol.1978.sp012544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner J. R., Stephens J. A. Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol. 1982 Dec;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton P. A., Morton H. B. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980 May 22;285(5762):227–227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Tanaka R., Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971 Nov;34(6):1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]



