Abstract
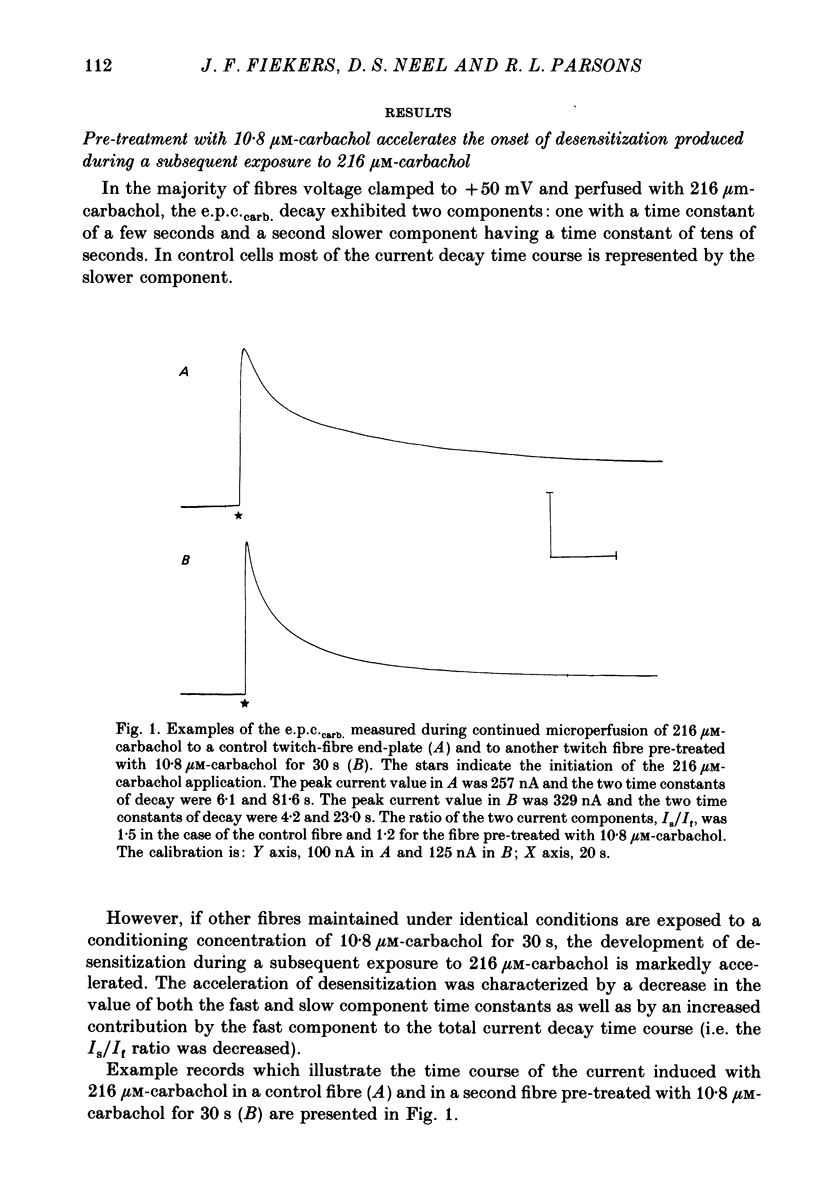
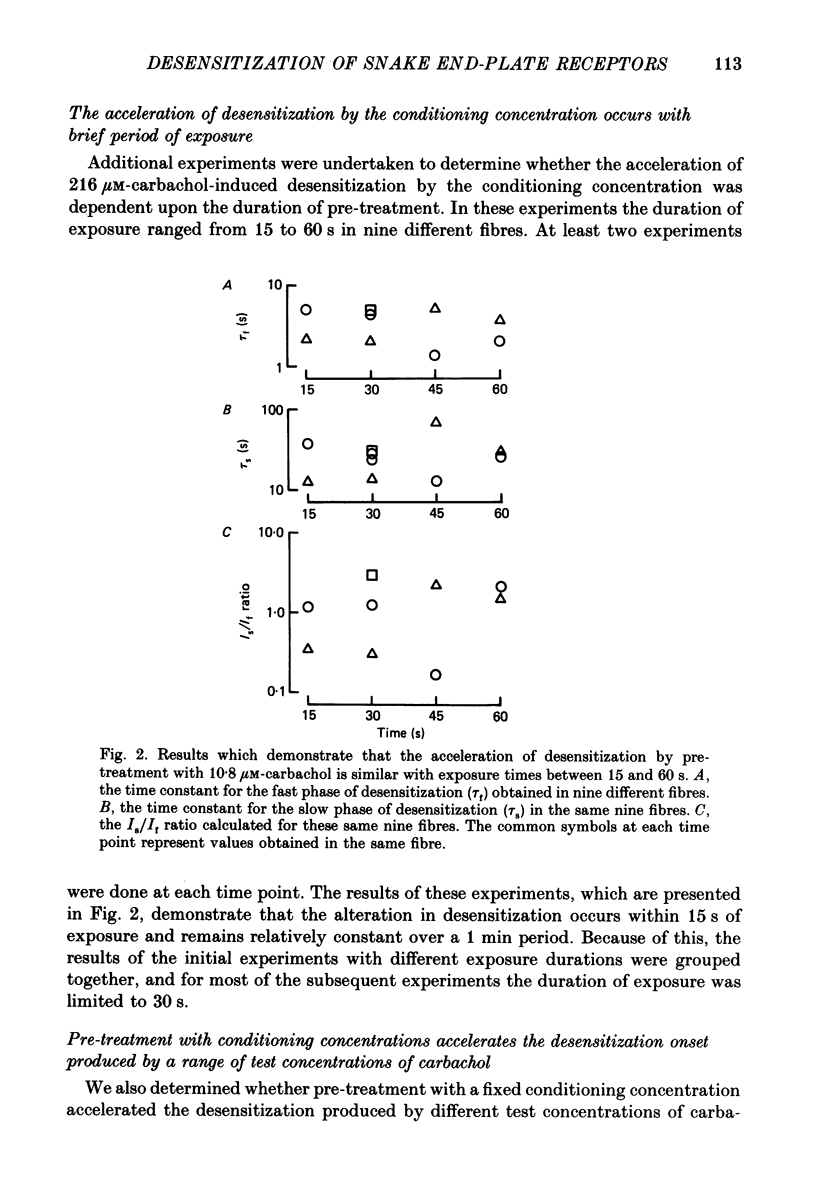
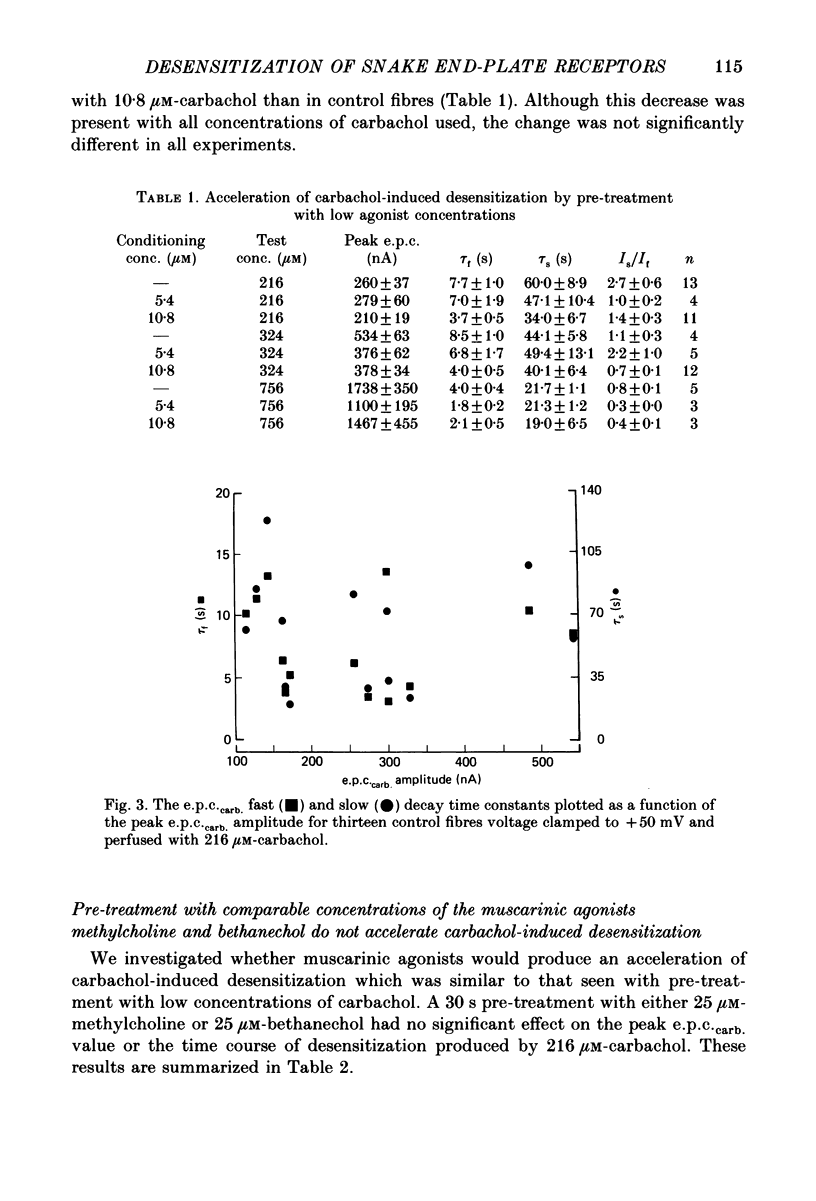
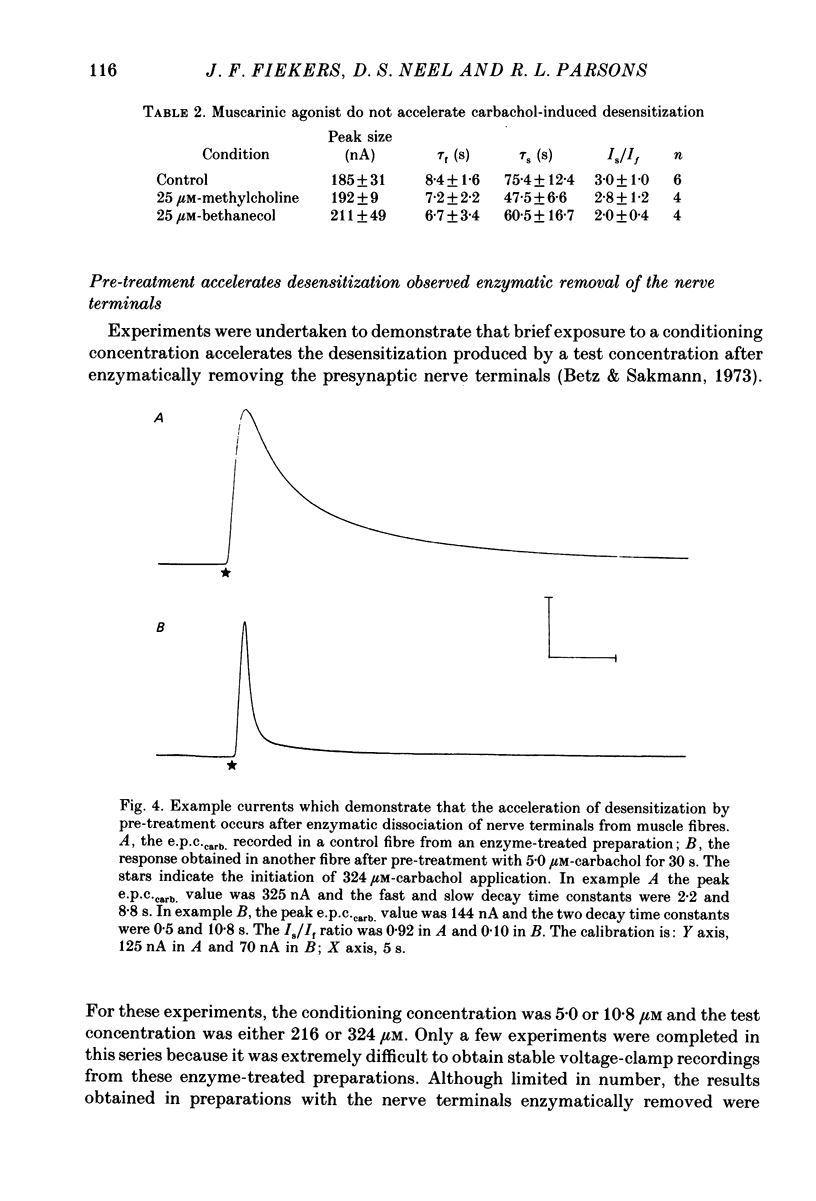
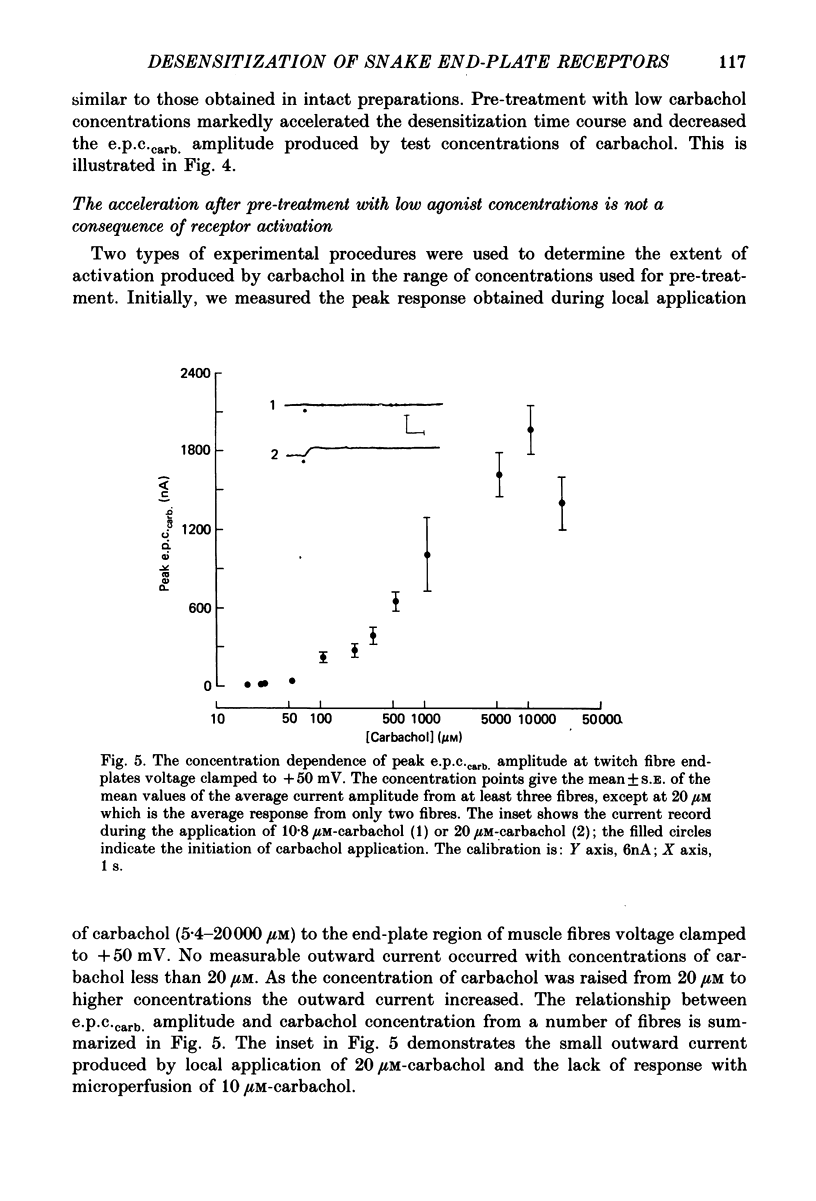
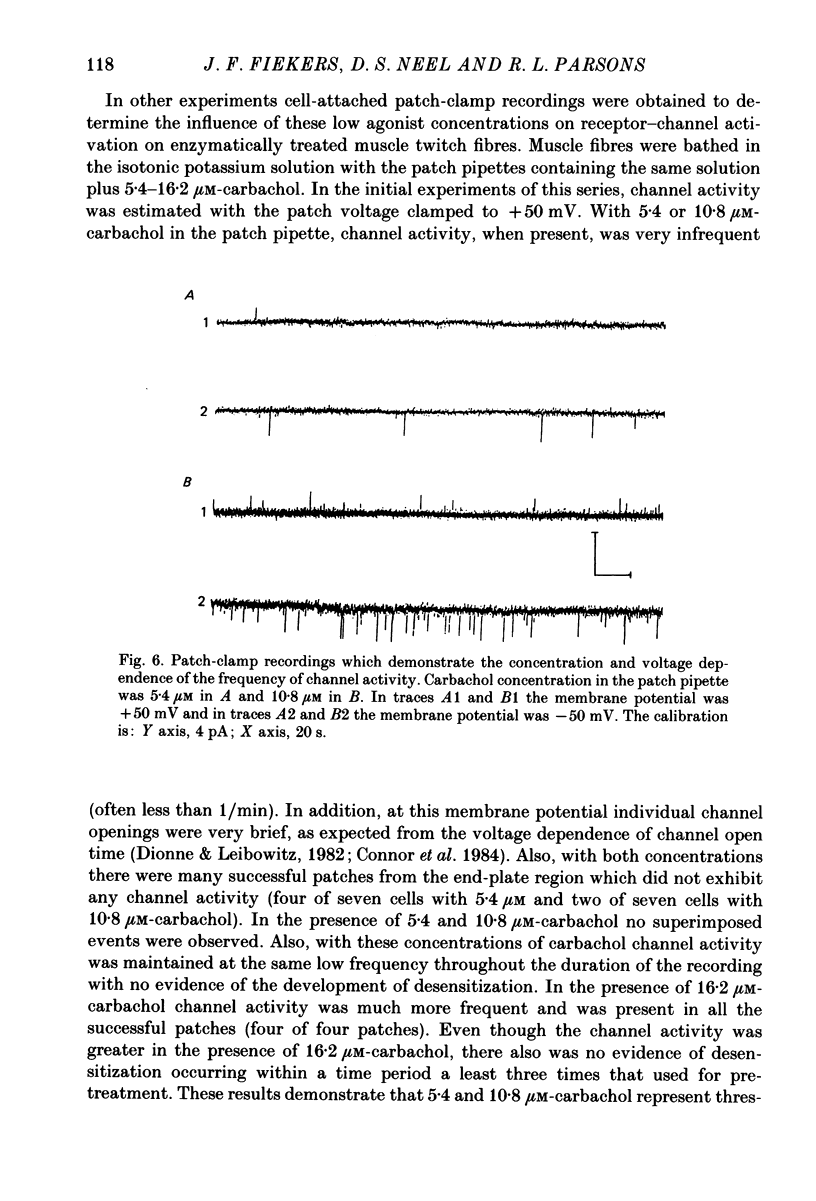
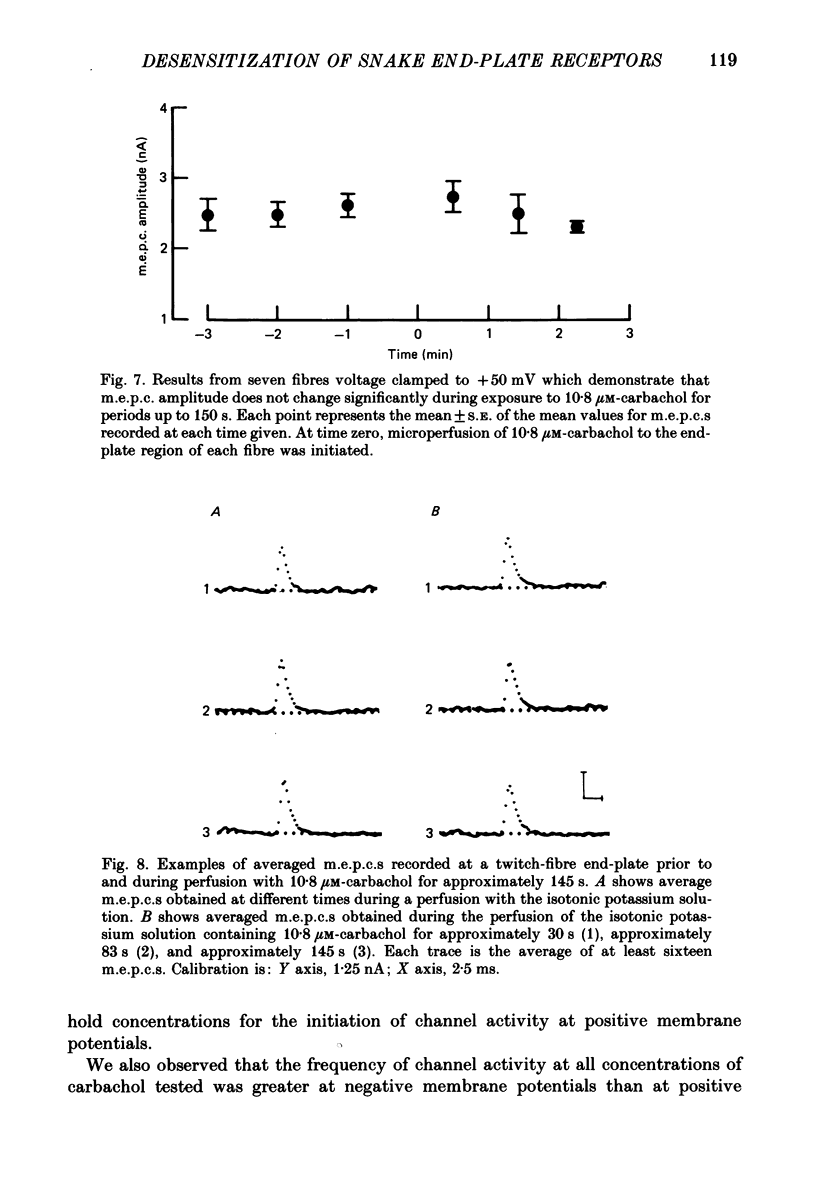
1. The kinetics of carbachol-induced desensitization have been studied in snake twitch-muscle fibres maintained in an isotonic potassium propionate solution and voltage clamped to +50 mV. 2. Microperfusion of carbachol (162-756 microM) induces a transient outward current which peaks within a few seconds and then slowly decays towards the base line. The time course of current decay estimates the time course of desensitization onset. 3. Brief exposure (30 s) to a 'conditioning' concentration of agonist (10.8 microM) accelerates the desensitization onset produced by exposure to higher 'test' concentrations of agonist (162-756 microM). 4. The acceleration of desensitization by pre-treatment with 10.8 microM-carbachol was independent of the duration of exposure between 15 and 60 s. This observation indicated that the mechanism responsible for the alteration in desensitization kinetics by treatment with 10.8 microM-carbachol differed from that responsible for the time-dependent development of desensitization produced in the presence of higher carbachol concentrations. 5. Pre-treatment with the muscarinic agonists, methylcholine and bethanechol, did not accelerate 216 microM-carbachol-induced desensitization, suggesting that the alteration of desensitization kinetics by pre-treatment was specific for nicotinic agonists. 6. The conditioning concentrations of carbachol (5.4-10.8 microM) produced no measurable outward current in muscle fibres voltage clamped to +50 mV. Further, in patch-clamp recordings it was observed that, with these concentrations of carbachol, there was no channel activity in many successful patches voltage clamped to +50 mV and, when present, the frequency of channel activity was very low. These results demonstrated that the alteration in desensitization was not the consequence of significant amounts of either receptor activation or desensitization produced by the conditioning concentration. 7. Exposure to 10.8 microM-carbachol for periods of up to 150 s did not change the amplitude of miniature end-plate currents recorded at end-plates voltage clamped to +50 mV. These results also demonstrated that the acceleration of desensitization by pre-treatment with conditioning concentrations of agonist was not due to partial desensitization occurring during the pre-treatment period. 8. Our results are consistent with the view that there are distinct populations of agonist binding sites on the acetylcholine receptor which separately regulate desensitization and channel opening.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Ikeda S. R., Brookes N., Pascuzzo G. J., Rickett D. L., Albuquerque E. X. The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. II. Patch clamp studies. Mol Pharmacol. 1984 Jan;25(1):102–112. [PubMed] [Google Scholar]
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Betz W., Sakmann B. Effects of proteolytic enzymes on function and structure of frog neuromuscular junctions. J Physiol. 1973 May;230(3):673–688. doi: 10.1113/jphysiol.1973.sp010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. A., Fiekers J. F., Neel D. S., Parsons R. L., Schnitzler R. M. Comparison of cholinergic activation and desensitization at snake twitch and slow muscle fibre end-plates. J Physiol. 1984 Jun;351:657–674. doi: 10.1113/jphysiol.1984.sp015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. Nicotinic acetylcholine receptor contains multiple binding sites: evidence from binding of alpha-dendrotoxin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6646–6650. doi: 10.1073/pnas.83.17.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. M., Raftery M. A. Activation and desensitization of Torpedo acetylcholine receptor: evidence for separate binding sites. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6757–6761. doi: 10.1073/pnas.79.22.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Fast desensitization of the nicotinic receptor at the mouse neuromuscular junction. Br J Pharmacol. 1982 Nov;77(3):395–404. doi: 10.1111/j.1476-5381.1982.tb09311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Conti-Tronconi B. M., Dunn S. M., Crawford R. D., Middlemas D. The nicotinic acetylcholine receptor: its structure, multiple binding sites, and cation transport properties. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 2):S34–S51. doi: 10.1016/0272-0590(84)90136-2. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A. A comparative study of the activation of the cholinergic receptor by various agonists. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):241–251. doi: 10.1098/rspb.1983.0037. [DOI] [PubMed] [Google Scholar]


