Abstract
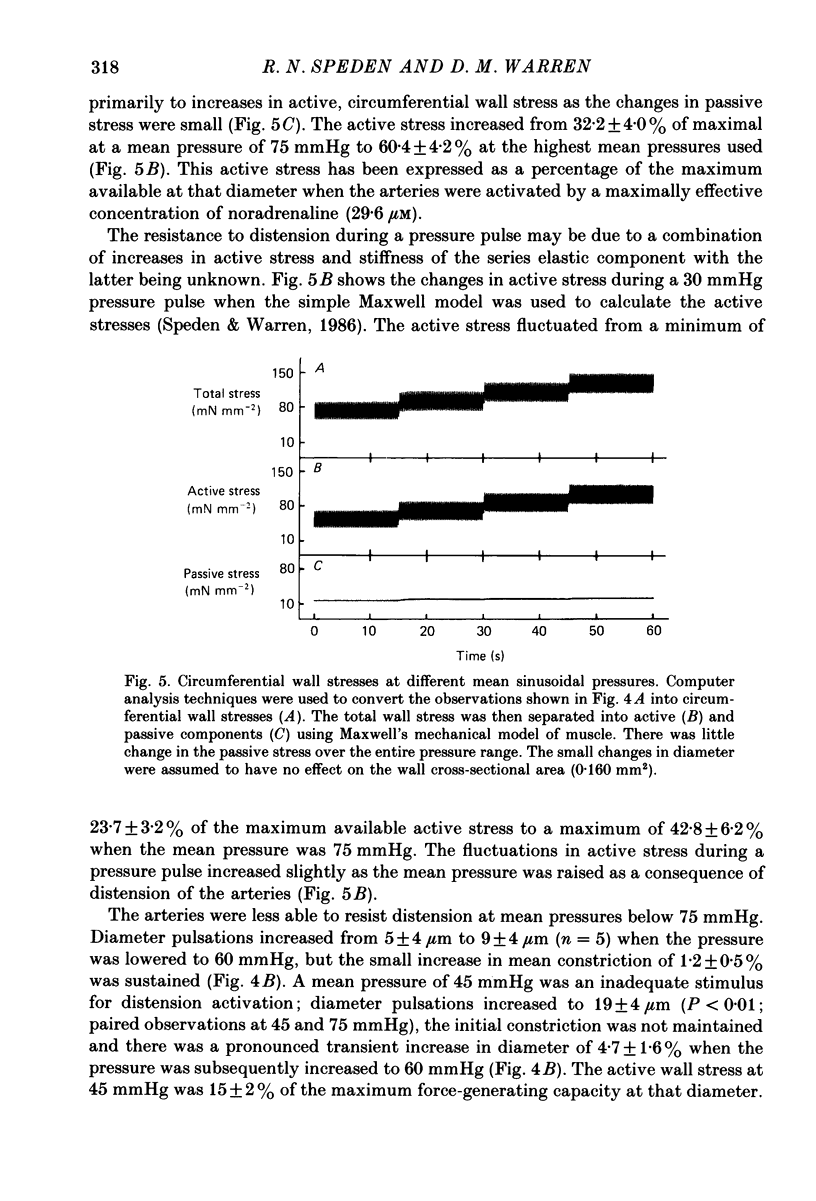
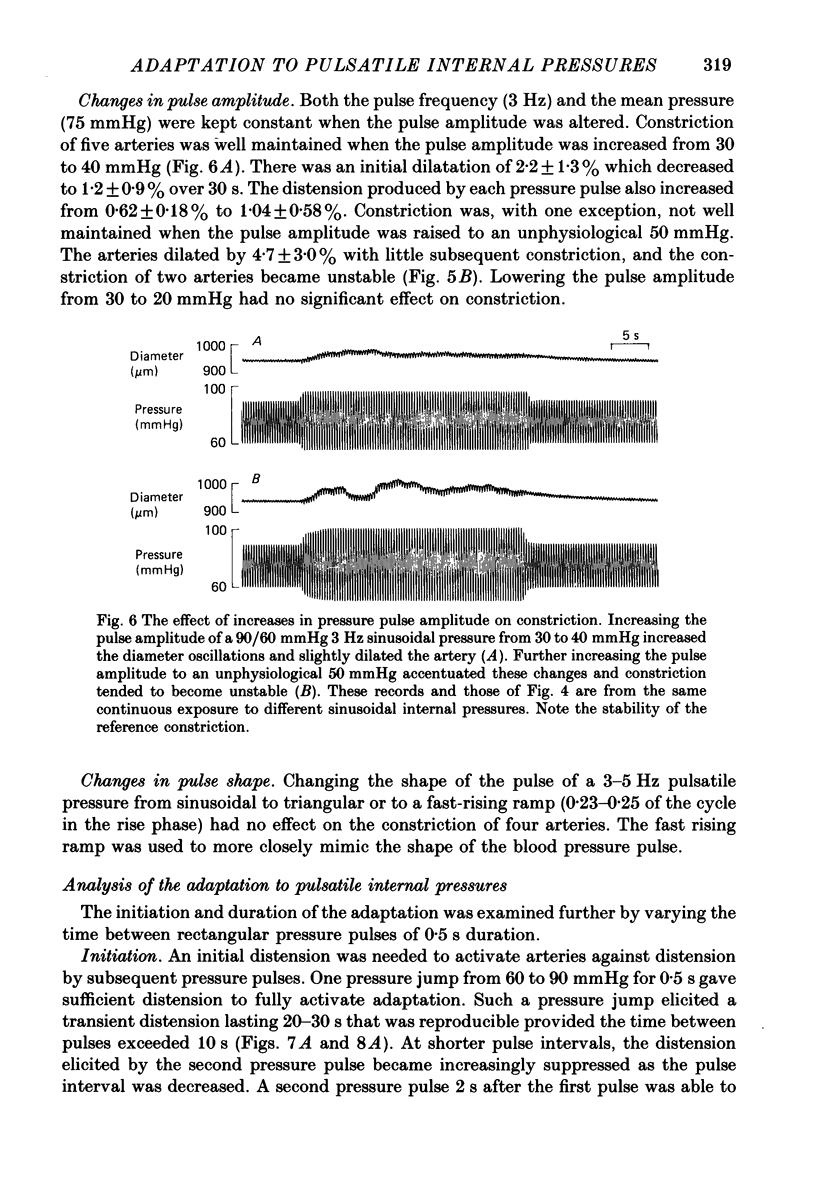
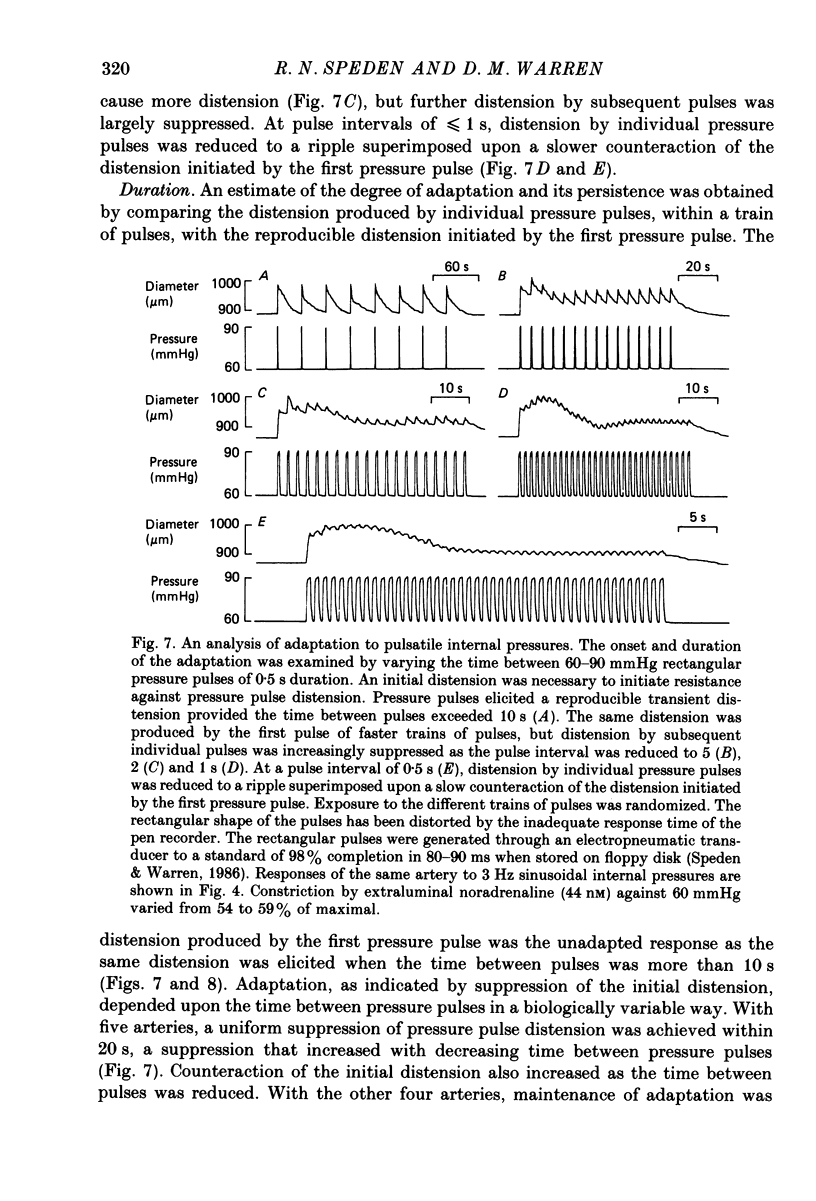
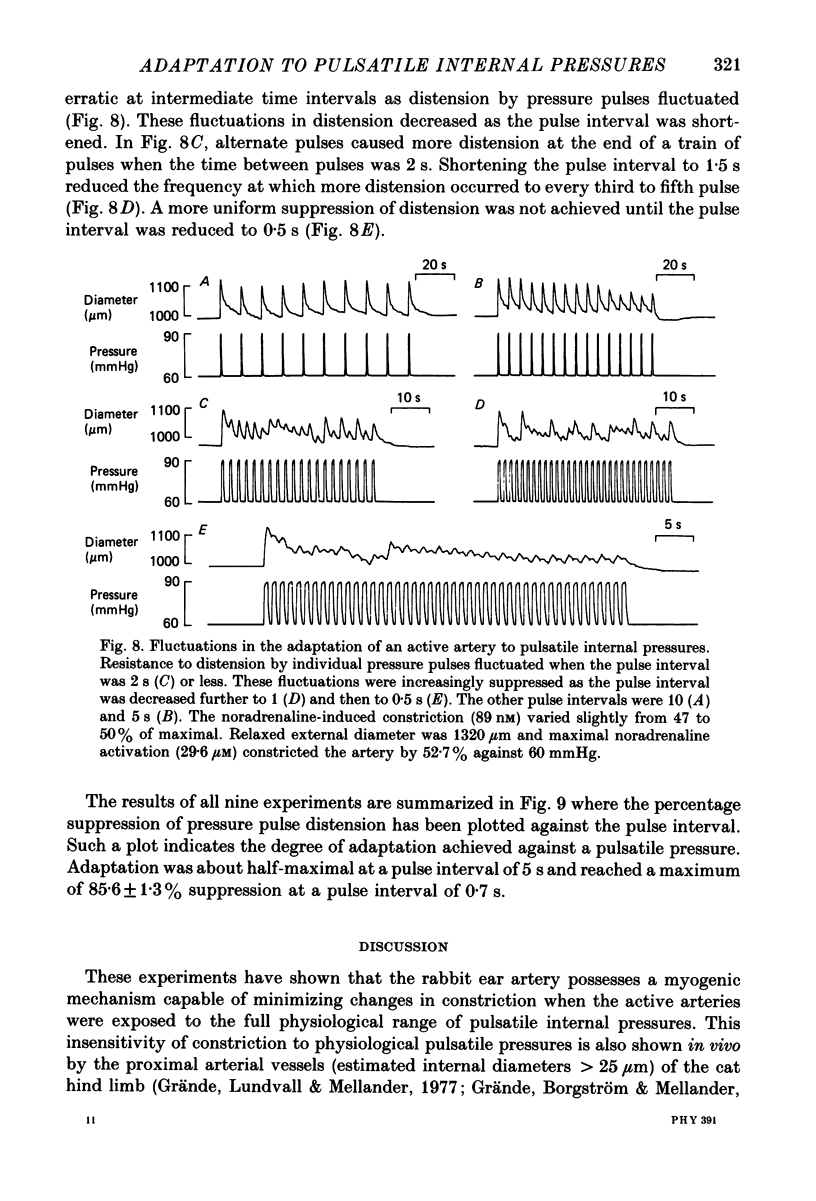
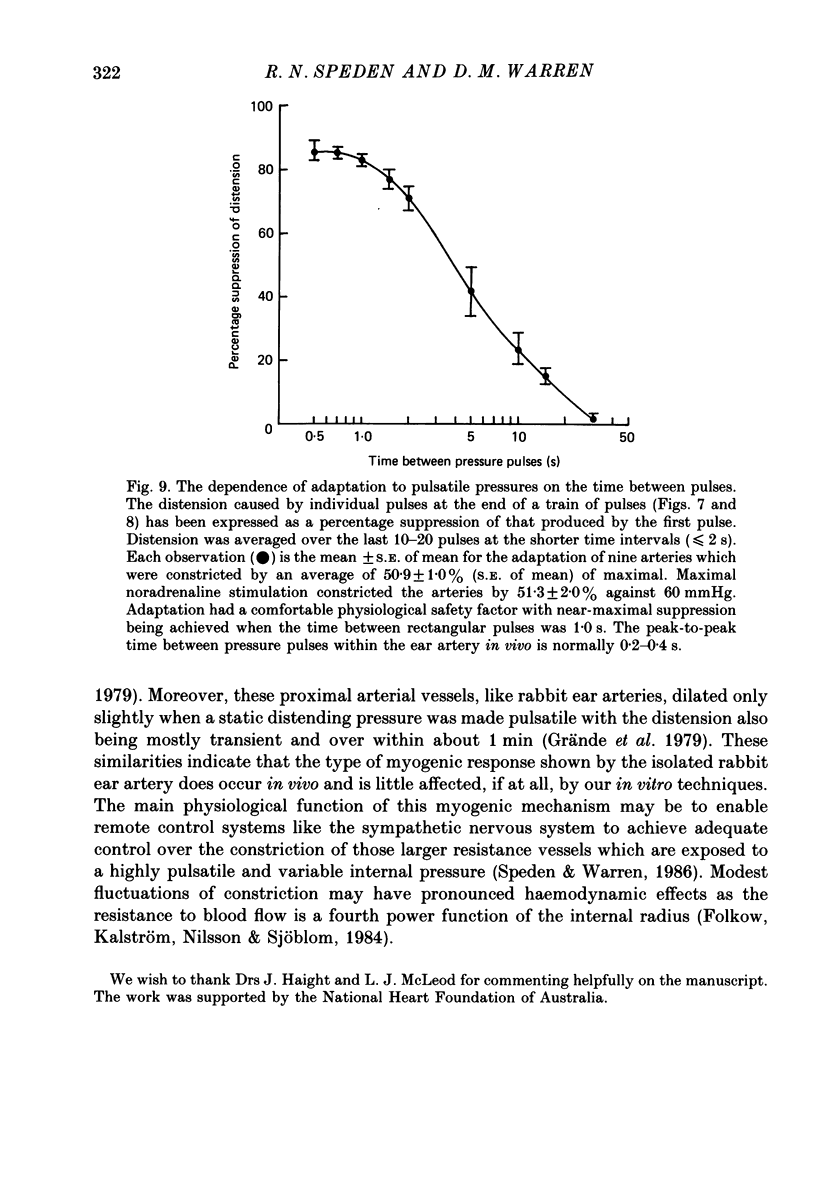
1. The effect of sinusoidal internal pressures on the constriction of in vitro, pressurized segments of ear arteries from rabbits has been examined. All arteries were constricted against a static transmural pressure of 60 mmHg to 35-60% of maximal, using extraluminal noradrenaline, before being exposed to the sinusoidal pressures. 2. There was a short period of adaptation when active arteries were first exposed to physiological pulsatile pressures. This adaptation had two components: a small, largely transient distension, lasting about 1 min, and sustained suppression of the distension produced by individual pressure pulses. 3. Constriction of adapted arteries was insensitive to physiological changes in pulse frequency (3-5 Hz), mean pulsatile pressure (60-120 mmHg), pulse amplitude (20-40 mmHg) and to alterations in pulse shape (sinusoidal, triangular and ramp). Over-all distension was restricted to 3.6 +/- 1.0% (S.D. of an observation) when the mean of a 3 Hz sinusoidal pressure of 30 mmHg amplitude was increased in steps from 75 to either 115 or 120 mmHg. 4. An initial distension was needed to initiate suppression of pressure pulse distension. Distension by individual pressure pulses, within a train of rectangular 60-90 mmHg pulses of 0.5 s duration, was maximally suppressed (85.6 +/- 1.3%; S.E. of mean, n = 9) at a pulse interval of 0.7 s. 5. Active ear arteries possess a myogenic mechanism capable of minimizing changes in constriction over the full physiological range of pulsatile internal pressures.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folkow B., Karlström G., Nilsson H., Sjöblom N. How do changes in diameter at the precapillary level affect cardiovascular function? J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S280–S288. doi: 10.1097/00005344-198406002-00002. [DOI] [PubMed] [Google Scholar]
- Grände P. O., Borgström P., Mellander S. On the nature of basal vascular tone in cat skeletal muscle and its dependence on transmural pressure stimuli. Acta Physiol Scand. 1979 Dec;107(4):365–376. doi: 10.1111/j.1748-1716.1979.tb06487.x. [DOI] [PubMed] [Google Scholar]
- Grände P. O., Lundvall J., Mellander S. Evidence for a rate-sensitive regulatory mechanism in myogenic microvascular control. Acta Physiol Scand. 1977 Apr;99(4):432–447. doi: 10.1111/j.1748-1716.1977.tb10396.x. [DOI] [PubMed] [Google Scholar]
- Ludbrook J., Graham W. F., Potocnik S. J. Acute deletion of arterial baroreceptor input in the conscious rabbit. Aust J Exp Biol Med Sci. 1985 Apr;63(Pt 2):231–240. doi: 10.1038/icb.1985.26. [DOI] [PubMed] [Google Scholar]
- Speden R. N. Active reactions of the rabbit ear artery to distension. J Physiol. 1984 Jun;351:631–643. doi: 10.1113/jphysiol.1984.sp015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speden R. N., Ryan A. T. Constriction of ear arteries from normotensive and renal hypertensive rabbits against different transmural pressures. Blood Vessels. 1982;19(5):247–262. doi: 10.1159/000158391. [DOI] [PubMed] [Google Scholar]
- Speden R. N., Warren D. M. The interaction between noradrenaline activation and distension activation of the rabbit ear artery. J Physiol. 1986 Jun;375:283–302. doi: 10.1113/jphysiol.1986.sp016117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. W., Traugott F. M., Quail A. W. Central nervous system 5-hydroxytryptamine and noradrenaline specificity of ear vascular and ventilation reflexes in thermoregulating rabbits. J Auton Nerv Syst. 1985 Feb-Mar;12(2-3):131–144. doi: 10.1016/0165-1838(85)90056-6. [DOI] [PubMed] [Google Scholar]


