Abstract
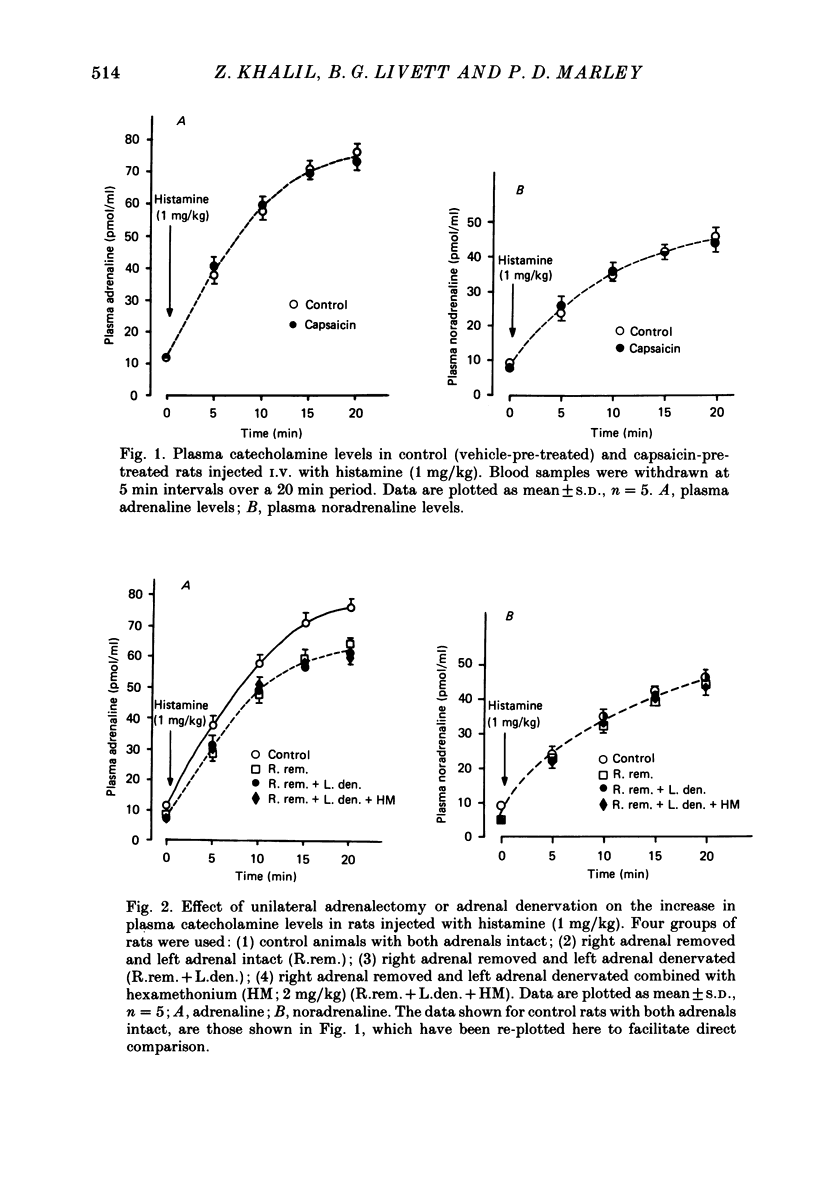
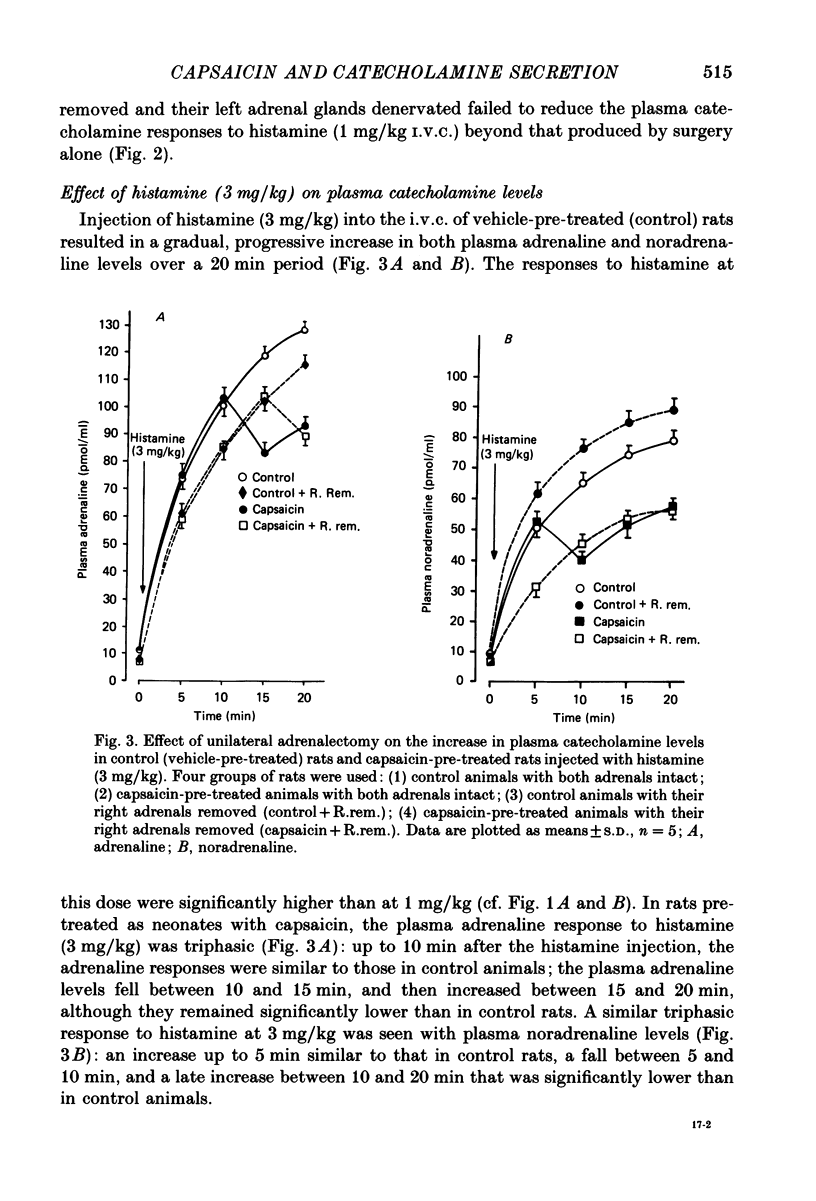
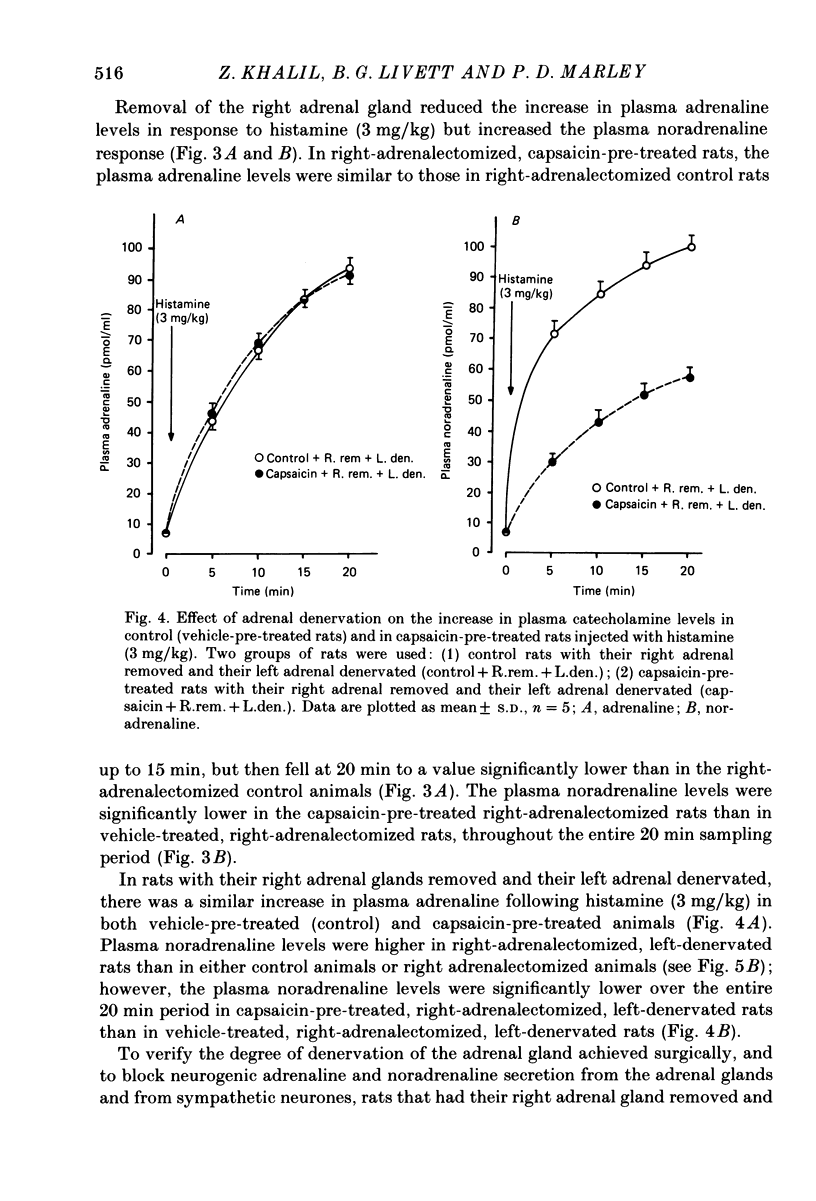
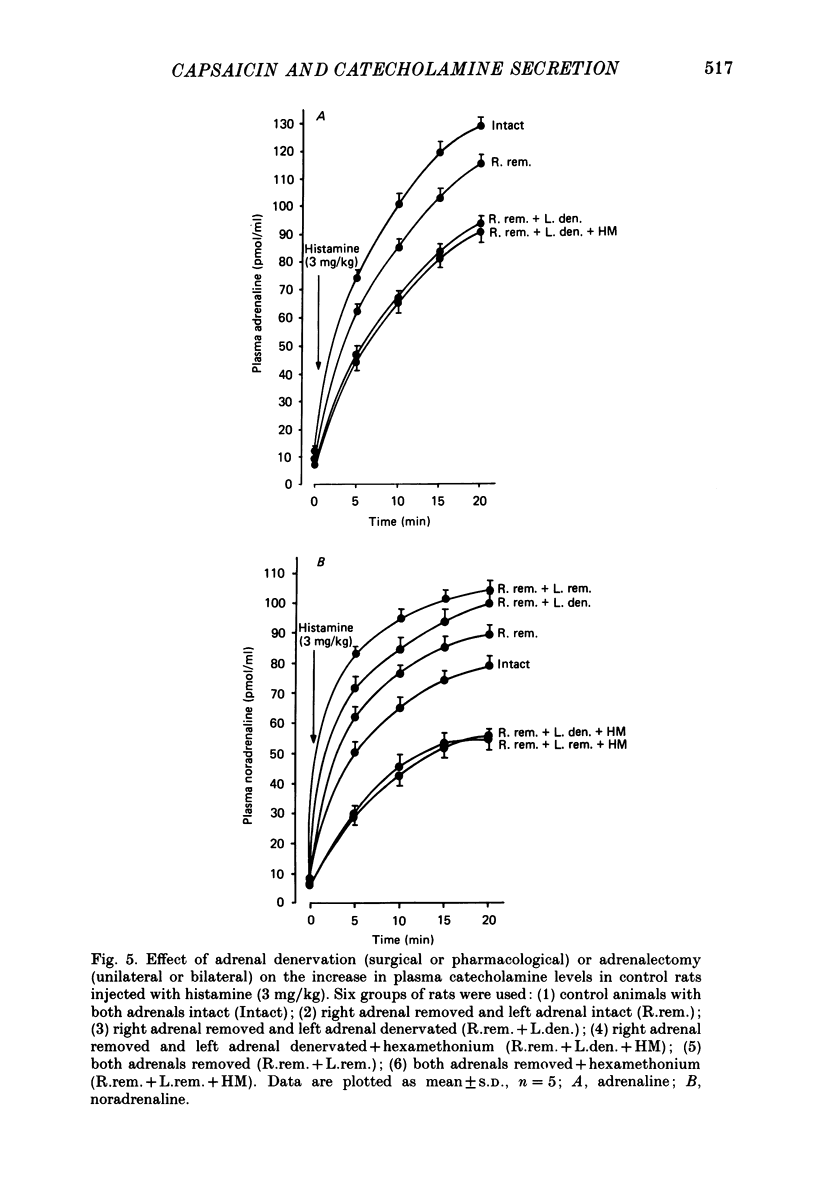
1. We have studied the mechanism of catecholamine secretion induced by histamine from the adrenal medulla and sympathetic noradrenergic neurones in the rat, and the role of capsaicin-sensitive sensory nerves in this secretion. 2. Histamine at a dose of 1 mg/kg induced adrenaline and noradrenaline secretion by a non-neurogenic mechanism. In contrast, at a dose of 3 mg/kg it induced adrenaline and noradrenaline secretion by both non-neurogenic and neurogenic mechanisms. 3. The adrenaline released in response to histamine at 3 mg/kg was exclusively of adrenal origin whereas the noradrenaline released was of non-adrenal origin (most probably noradrenergic sympathetic nerves). As with its action on the adrenal, histamine induced noradrenaline secretion from these extra-adrenal tissues by both neurogenic and non-neurogenic mechanisms. 4. When adrenaline secretion from the adrenal gland was impaired by adrenal denervation and/or adrenalectomy, the plasma noradrenaline secretion was increased. This is most probably due to compensation from the rest of the sympathetic nervous system. This compensatory increase in noradrenaline was abolished by hexamethonium, which indicates that it was mediated by a cholinergic mechanism. 5. Pre-treatment of rats as neonates subcutaneously with capsaicin (a selective neurotoxin for certain sensory nerves) at a dose of 50 mg/kg, had no effect on the non-neurogenic secretion of catecholamine induced by histamine. In contrast, capsaicin pre-treatment abolished the neurogenic catecholamine secretion in response to histamine as well as the neurogenic compensatory increase in plasma noradrenaline levels that occurred when adrenaline secretion by the adrenal gland was impaired. 6. In the present study, by using histamine as a form of stress, we have been able to provide evidence not only (a) to confirm a role for capsaicin-sensitive sensory fibres in modulating neurogenic adrenaline secretion from the adrenal gland, but also (b) to suggest a similar role for these fibres in modulating neurogenic noradrenaline release from sympathetic noradrenergic nerves in response to histamine and in response to impairment of adrenaline secretion by the adrenal gland.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Kojima M., Koketsu K. Substance P modulates the sensitivity of the nicotinic receptor in amphibian cholinergic transmission. Br J Pharmacol. 1983 Sep;80(1):123–131. doi: 10.1111/j.1476-5381.1983.tb11057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T., Hamamoto T., Kurosawa M., Sato A. Response of adrenal efferent nerve activity to noxious stimulation of the skin. Neurosci Lett. 1980 Apr;17(1-2):131–135. doi: 10.1016/0304-3940(80)90074-9. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W. Substance P and Renshaw cells: a new concept of inhibitory synaptic interactions. J Physiol. 1977 Oct;272(1):105–119. doi: 10.1113/jphysiol.1977.sp012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P., Livett B. G. Substance P protects against desensitization of the nicotinic response in isolated adrenal chromaffin cells. J Neurochem. 1984 Mar;42(3):618–627. doi: 10.1111/j.1471-4159.1984.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Chahl L. A., Cross R. B. The nature of the pressor response to carotid artery occlusion in the hexamethonium-treated rat. Clin Exp Pharmacol Physiol. 1978 Sep-Oct;5(5):495–502. doi: 10.1111/j.1440-1681.1978.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Dray A. Substance P and opiate receptors. Nature. 1977 Jul 28;268(5618):351–352. doi: 10.1038/268351a0. [DOI] [PubMed] [Google Scholar]
- Douglas J. S., Dennis M. W., Ridgway P., Bouhuys A. Airway constriction in guinea pigs: interaction of histamine and autonomic drugs. J Pharmacol Exp Ther. 1973 Jan;184(1):169–179. [PubMed] [Google Scholar]
- Fisher L. A., Kikkawa D. O., Rivier J. E., Amara S. G., Evans R. M., Rosenfeld M. G., Vale W. W., Brown M. R. Stimulation of noradrenergic sympathetic outflow by calcitonin gene-related peptide. Nature. 1983 Oct 6;305(5934):534–536. doi: 10.1038/305534a0. [DOI] [PubMed] [Google Scholar]
- Gamse R., Leeman S. E., Holzer P., Lembeck F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):140–148. doi: 10.1007/BF00500070. [DOI] [PubMed] [Google Scholar]
- Gibson S. J., Polak J. M., Bloom S. R., Sabate I. M., Mulderry P. M., Ghatei M. A., McGregor G. P., Morrison J. F., Kelly J. S., Evans R. M. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci. 1984 Dec;4(12):3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth M. A. The distribution of vasoactive intestinal peptide in the rat adrenal cortex and medulla. J Auton Nerv Syst. 1984 Nov;11(3):269–283. doi: 10.1016/0165-1838(84)90041-9. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Fahrenkrug J. Immunohistochemical evidence for a local VIP-ergic neuron system in the adrenal gland of the rat. Acta Physiol Scand. 1981 Dec;113(4):575–576. doi: 10.1111/j.1748-1716.1981.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Hökfelt T., Lundberg J. M., Kiraly E., Halász N., Nilsson G., Terenius L., Rehfeld J., Steinbusch H., Verhofstad A. Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrin/CCK, somatostatin, VIP, enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol. 1981 Dec;10(6):963–980. doi: 10.1007/BF01258524. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Király E. Sensory neurotoxins: chemically induced selective destruction of primary sensory neurons. Brain Res. 1981 Apr 6;210(1-2):83–89. doi: 10.1016/0006-8993(81)90886-6. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Livett B. G., Marley P. D. The role of sensory fibres in the rat splanchnic nerve in the regulation of adrenal medullary secretion during stress. J Physiol. 1986 Jan;370:201–215. doi: 10.1113/jphysiol.1986.sp015930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil Z., Marley P. D., Livett B. G. Elevation in plasma catecholamines in response to insulin stress is under both neuronal and nonneuronal control. Endocrinology. 1986 Jul;119(1):159–167. doi: 10.1210/endo-119-1-159. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lekić D. Substance P selectively blocks excitation of Renshaw cell by acetylcholine. Can J Physiol Pharmacol. 1977 Aug;55(4):958–961. doi: 10.1139/y77-129. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Reflex fall in blood pressure mediated by capsaicin-sensitive afferent fibers of the rat splanchnic nerve. Naunyn Schmiedebergs Arch Pharmacol. 1983 May;322(4):286–289. doi: 10.1007/BF00508344. [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Reit E. Further studies on the actions of peptides on the superior cervical ganglion and suprarenal medulla. Br J Pharmacol Chemother. 1966 Feb;26(2):444–460. doi: 10.1111/j.1476-5381.1966.tb01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila R. I., Diaugustine R. P., Hervonen A., Miller R. J. Distribution of [Met5]- and [Leu5]-enkephalin-, vasoactive intestinal polypeptide- and substance P-like immunoreactivities in human adrenal glands. Neuroscience. 1980;5(12):2247–2259. doi: 10.1016/0306-4522(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Kozousek V., Mizobe F., Dean D. M. Substance P inhibits nicotinic activation of chromaffin cells. Nature. 1979 Mar 15;278(5701):256–257. doi: 10.1038/278256a0. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Marley P. D. Effects of opioid peptides and morphine on histamine-induced catecholamine secretion from cultured, bovine adrenal chromaffin cells. Br J Pharmacol. 1986 Oct;89(2):327–334. doi: 10.1111/j.1476-5381.1986.tb10264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio J. E., Hunter J. C. Regional distribution of kassinin-like immunoreactivity in rat central and peripheral tissues and the effect of capsaicin. Brain Res. 1984 Jul 30;307(1-2):370–373. doi: 10.1016/0006-8993(84)90498-0. [DOI] [PubMed] [Google Scholar]
- Marley P., Livett B. G. Neuropeptides in the autonomic nervous system. CRC Crit Rev Clin Neurobiol. 1985;1(3):201–283. [PubMed] [Google Scholar]
- Matthews M. R., Cuello A. C. The origin and possible significance of substance P immunoreactive networks in the prevertebral ganglia and related structures in the guinea-pig. Philos Trans R Soc Lond B Biol Sci. 1984 Aug 14;306(1128):247–276. doi: 10.1098/rstb.1984.0087. [DOI] [PubMed] [Google Scholar]
- McQueen D. S. Effects of substance P on carotid chemoreceptor activity in the cat. J Physiol. 1980 May;302:31–47. doi: 10.1113/jphysiol.1980.sp013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobe F., Kozousek V., Dean D. M., Livett B. G. Pharmacological characterization of adrenal paraneurons: substance P and somatostatin as inhibitory modulators of the nicotinic response. Brain Res. 1979 Dec 14;178(2-3):555–566. doi: 10.1016/0006-8993(79)90714-5. [DOI] [PubMed] [Google Scholar]
- Role L. W., Leeman S. E., Perlman R. L. Somatostatin and substance P inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience. 1981;6(9):1813–1821. doi: 10.1016/0306-4522(81)90215-3. [DOI] [PubMed] [Google Scholar]
- Simasko S. M., Soares J. R., Weiland G. A. Structure-activity relationship for substance P inhibition of carbamylcholine-stimulated 22Na+ flux in neuronal (PC12) and non-neuronal (BC3H1) cell lines. J Pharmacol Exp Ther. 1985 Dec;235(3):601–605. [PubMed] [Google Scholar]
- Stallcup W. B., Patrick J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staszewska-Barczak J., Vane J. R. The release of catecholamines from the adrenal medulla by peptides. Br J Pharmacol Chemother. 1967 Aug;30(3):655–667. doi: 10.1111/j.1476-5381.1967.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacker A. Calcium-dependent presynaptic action of substance P at the frog neuromuscular junction. Nature. 1977 May 19;267(5608):268–270. doi: 10.1038/267268a0. [DOI] [PubMed] [Google Scholar]
- Steinacker A., Highstein S. M. Pre- and postsynaptic action of substance P at the Mauther fiber-giant fiber synapse in the hatchetfish. Brain Res. 1976 Sep 10;114(1):128–133. doi: 10.1016/0006-8993(76)91013-1. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. The action of histamine and pilocarpine on the superior cervical ganglion and the adrenal glands of the cat. Br J Pharmacol Chemother. 1954 Dec;9(4):481–487. doi: 10.1111/j.1476-5381.1954.tb00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashpal K., Gauthier S. G., Henry J. L. Substance P given intrathecally at the spinal T9 level increases adrenal output of adrenaline and noradrenaline in the rat. Neuroscience. 1985 Jun;15(2):529–536. doi: 10.1016/0306-4522(85)90231-3. [DOI] [PubMed] [Google Scholar]
- Yoshizaki T. Effect of histamine, bradykinin and morphine on adrenaline release from rat adrenal gland. Jpn J Pharmacol. 1973 Oct;23(5):695–699. doi: 10.1254/jjp.23.695. [DOI] [PubMed] [Google Scholar]


