Abstract
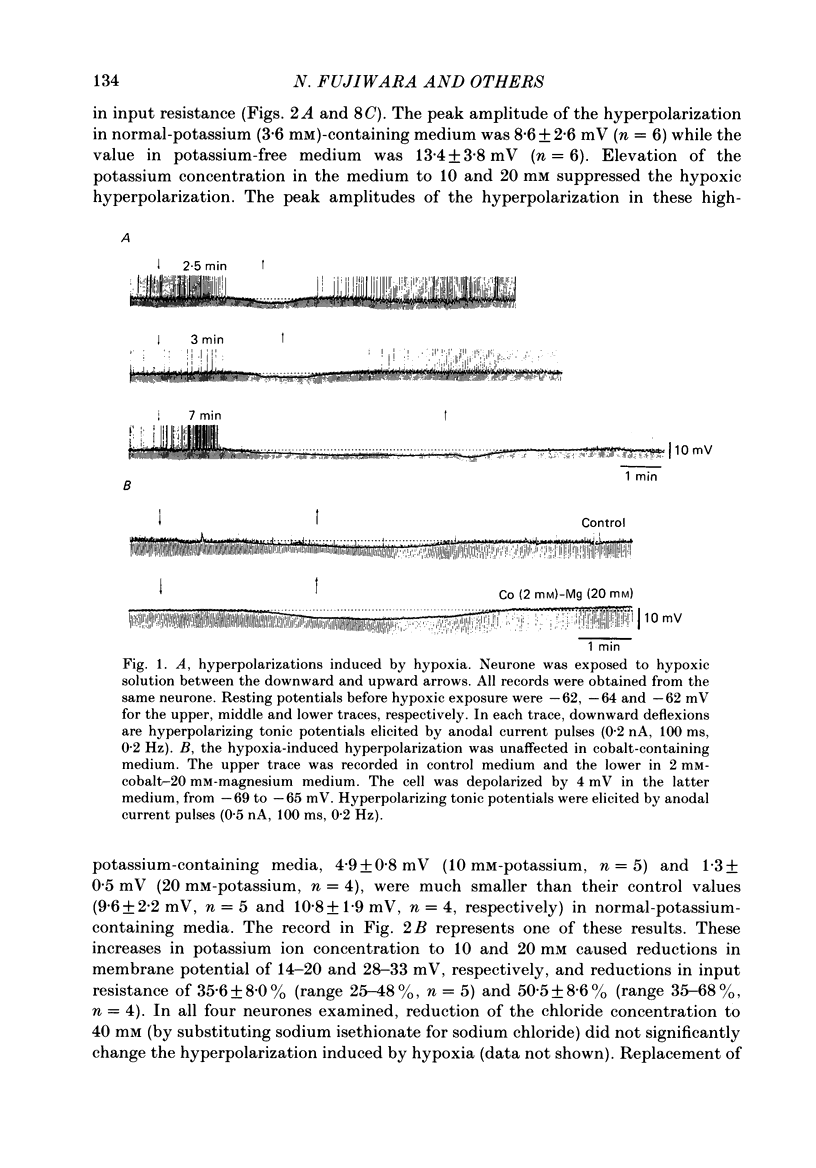
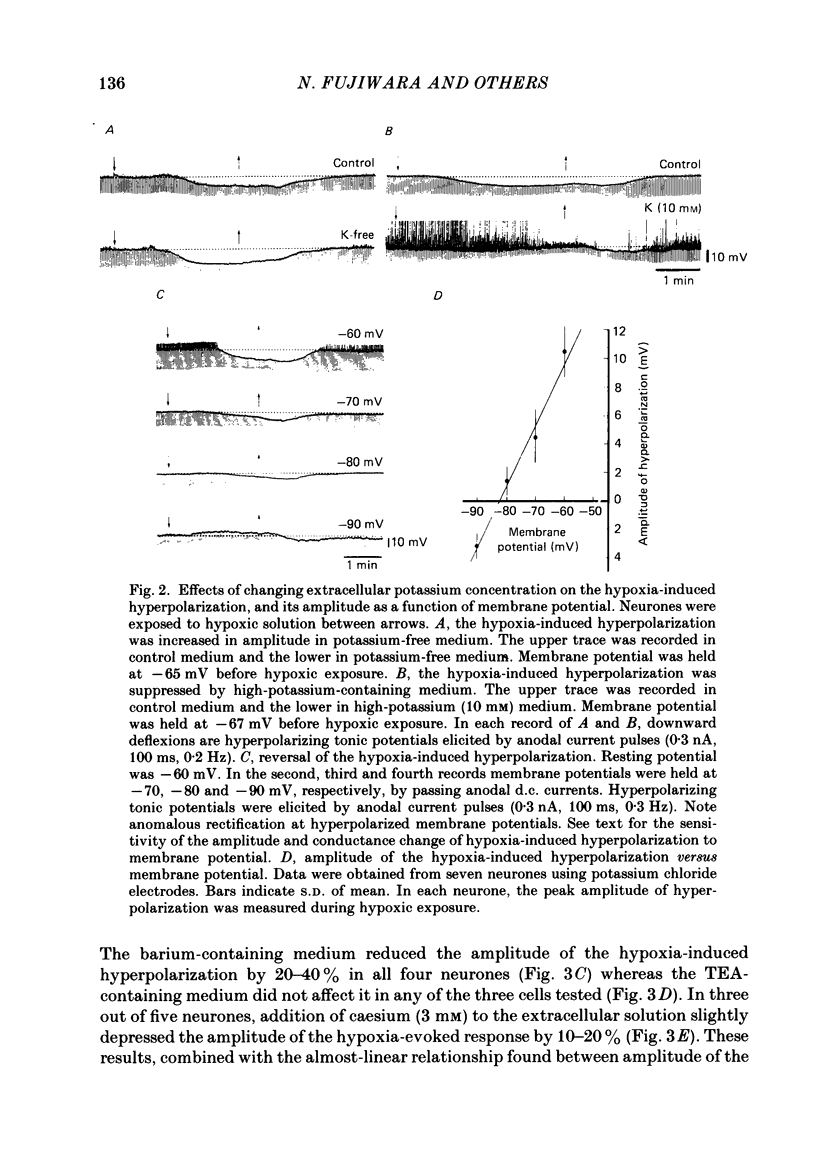
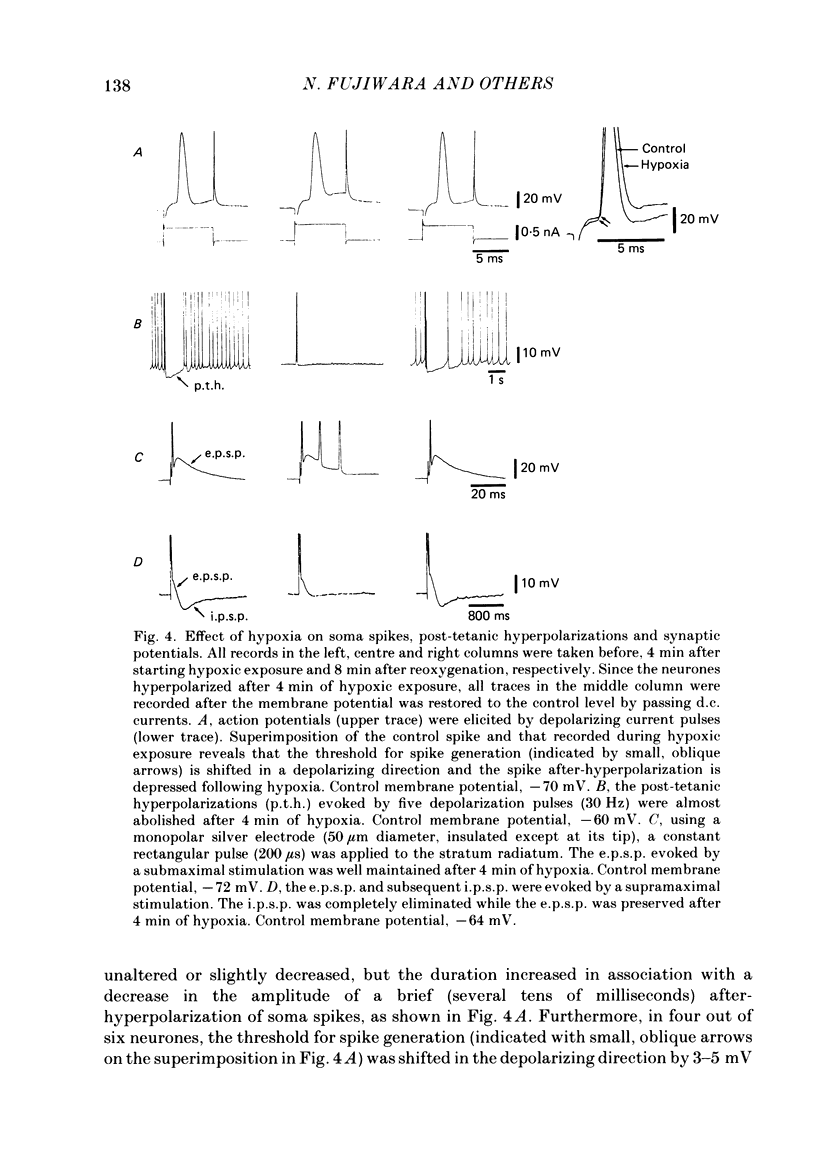
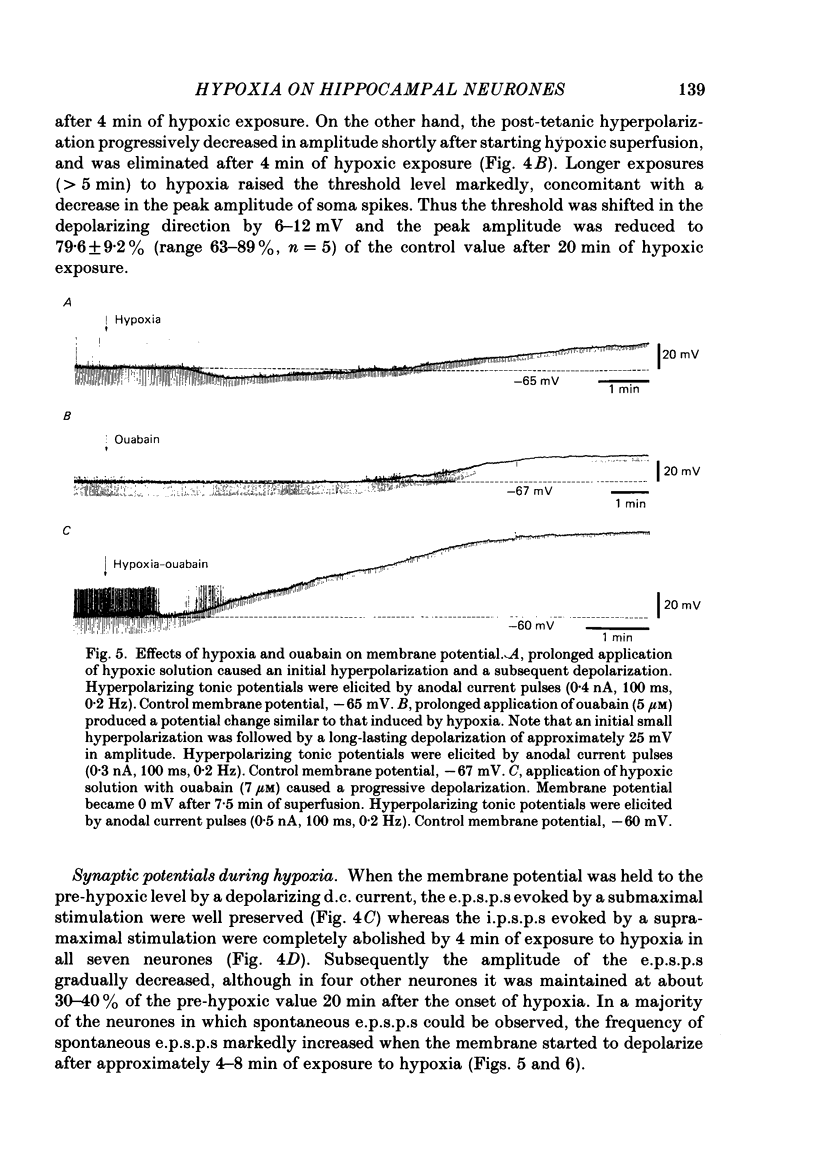
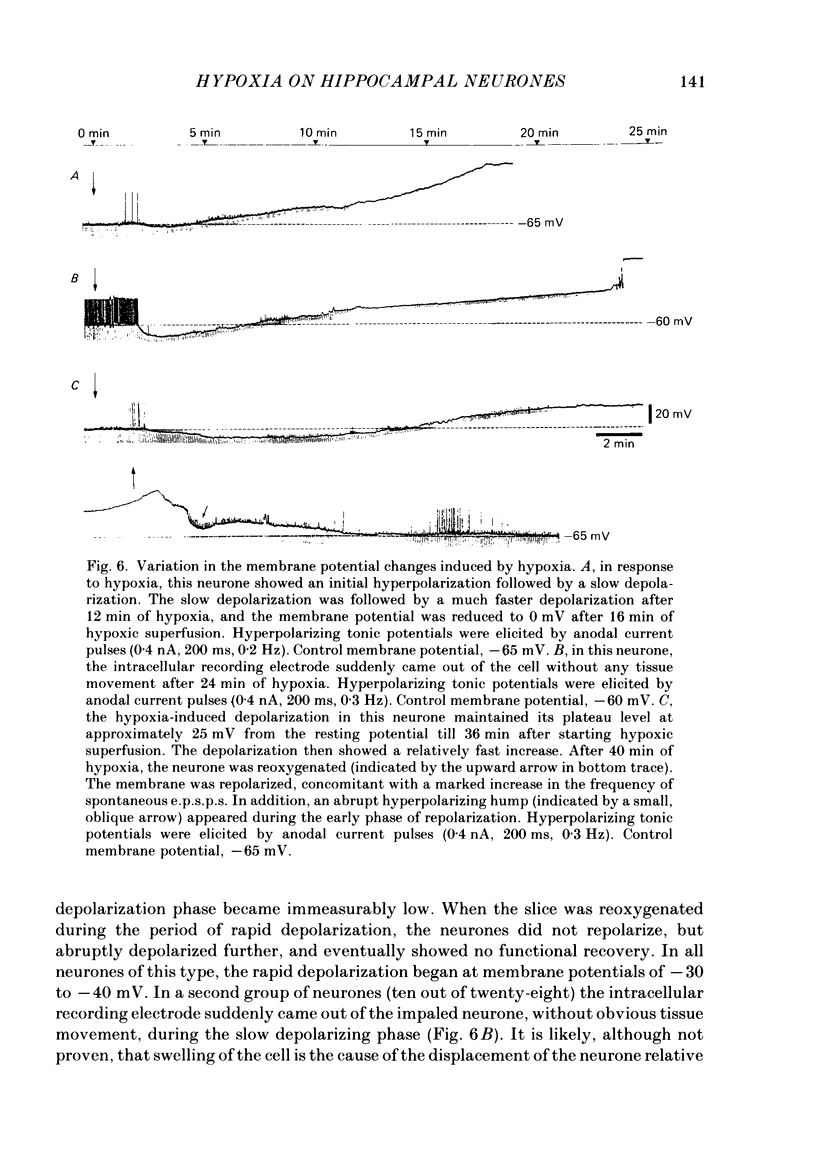
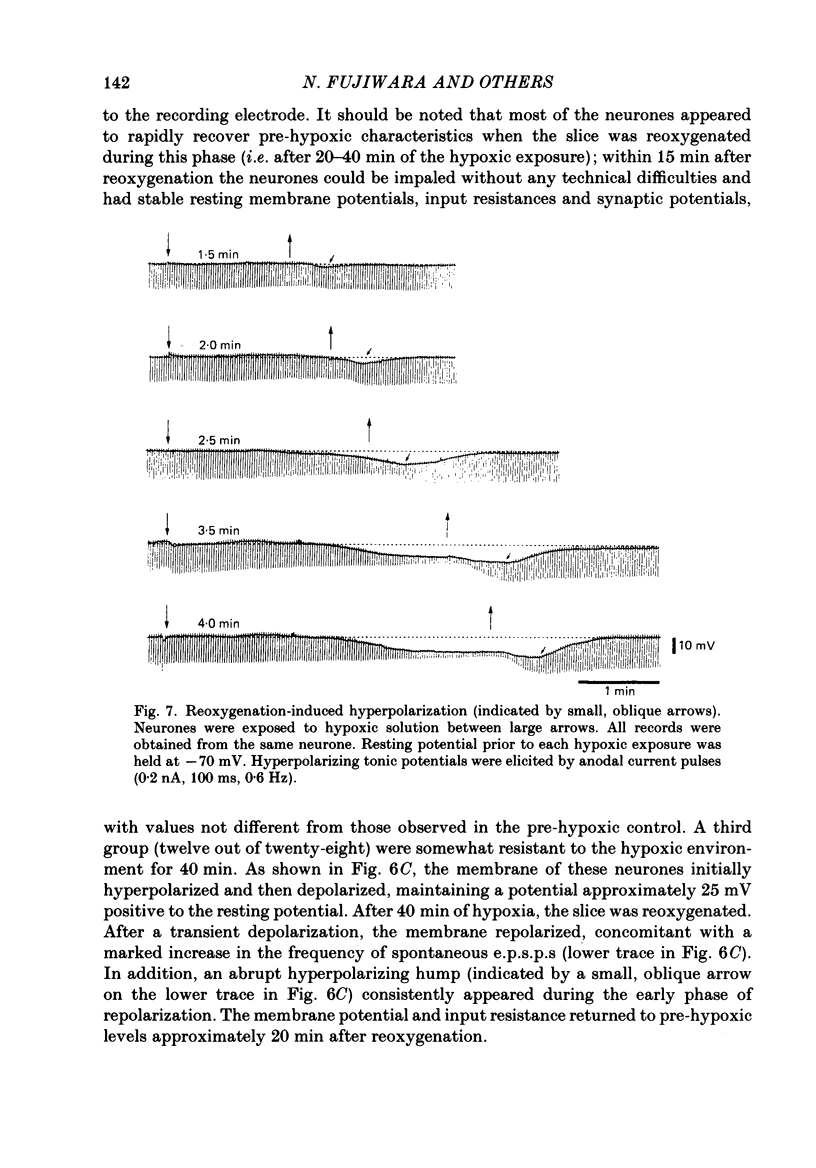
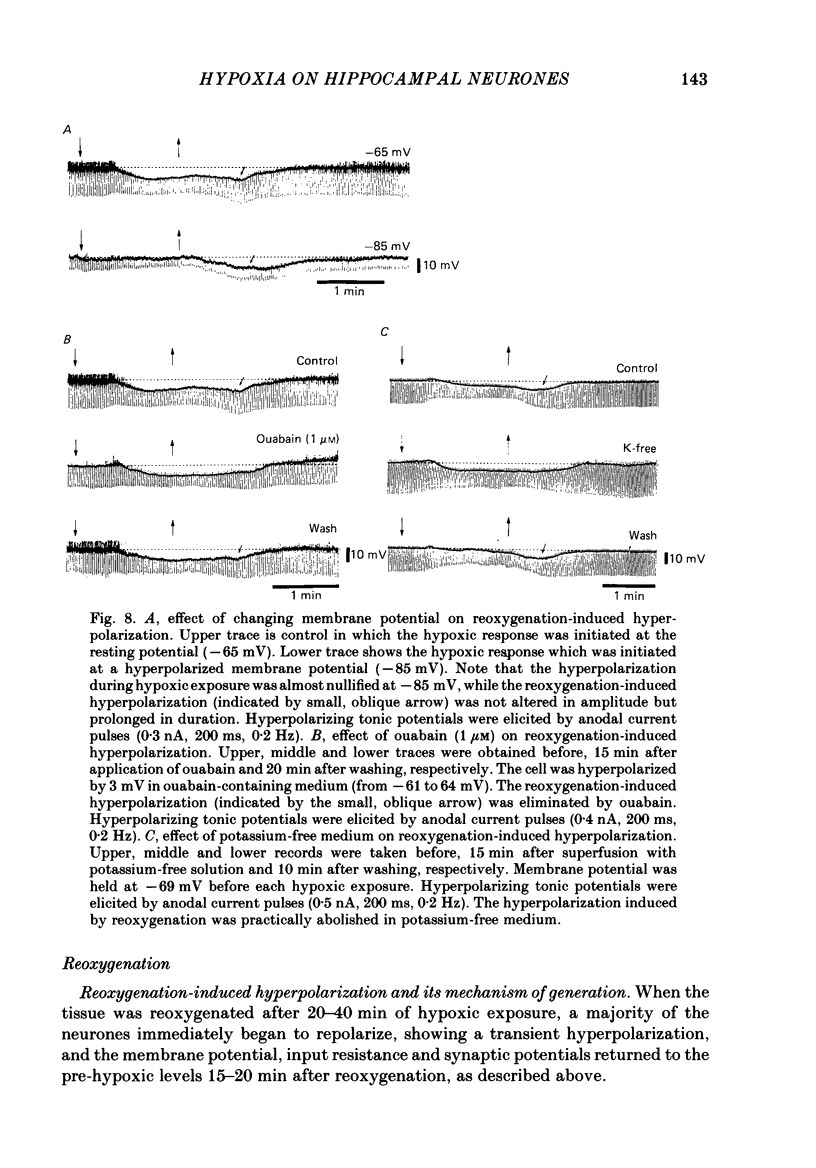
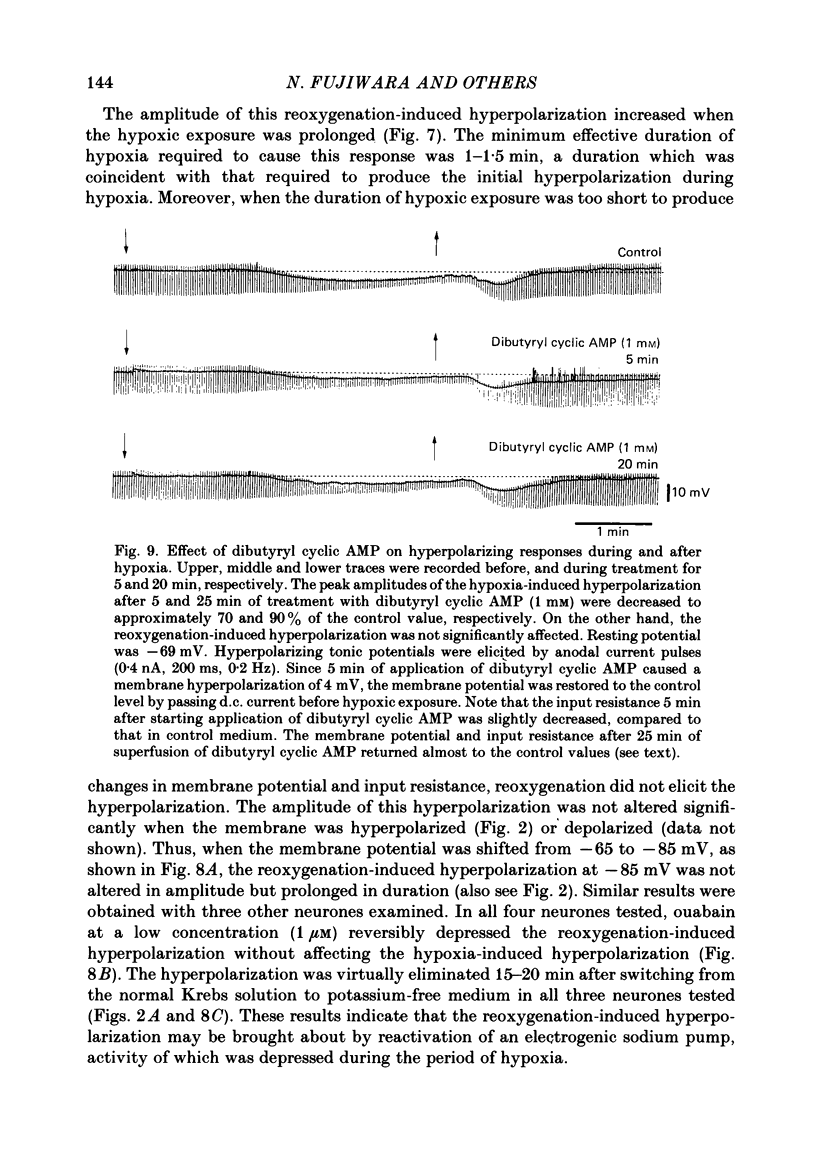
1. The effects of hypoxia on the rat hippocampal CA1 neurones in tissue slices of the rat brain were studied in vitro by intracellular recording. 2. In response to superfusion of a hypoxic medium equilibrated with 95% N2-5% CO2, a majority of the neurones showed a hyperpolarization of 5-15 mV in amplitude and 4-12 min in duration. The hyperpolarization was, in turn, followed by a slow depolarization which within 20 min of hypoxic exposure reached a plateau level of about 25 mV above the pre-hypoxic resting potential. Both the initial hyperpolarization and subsequent depolarization were associated with a reduction in membrane resistance. 3. The hyperpolarization reversed in polarity at a membrane potential of -83 mV. There was an almost linear relationship between amplitude of the hyperpolarization and membrane potential. The hyperpolarization was markedly enhanced in potassium-free media and was depressed in high-potassium solutions. 4. The hyperpolarization was not significantly affected by low-chloride or low-sodium medium or by solution containing tetraethylammonium (10 mM), 4-aminopyridine (1.5 mM) or caesium (3 mM). Moreover, intracellular injection of ethyleneglycol-bis-(beta-aminoethylether)N,N-tetraacetic acid (EGTA) did not alter the hyperpolarization. On the other hand, barium (0.5 mM)-containing medium reduced the amplitude of the hyperpolarization by 20-40%. 5. Superfusion of ouabain (5-7 microM)-containing medium in normoxic conditions produced hyperpolarizing and depolarizing responses similar to those elicited by hypoxic exposure. The slow depolarization was also mimicked by elevation of the extracellular potassium concentration to 10-20 mM. 6. Evoked i.p.s.p.s were abolished within 4 min of hypoxic exposure while evoked e.p.s.p.s were maintained for about 20 min of hypoxic superfusion. Soma spikes of the neurones elicited by a depolarizing pulse were also well preserved. Their threshold was, however, raised, concomitant with a decrease in the peak amplitude. 7. When the slice was reoxygenated after 20-40 min of hypoxic exposure, the neurones immediately began to repolarize and showed a transient hyperpolarization of 5-10 mV in amplitude and 1-2 min in duration. The membrane potential, input resistance and action potential returned to the pre-hypoxic levels after 15-20 min of reoxygenation. The amplitude of the reoxygenation-induced hyperpolarization was not significantly changed when the membrane was hyperpolarized or depolarized. The hyperpolarization was eliminated by potassium-free medium or solution containing ouabain (1 microM).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



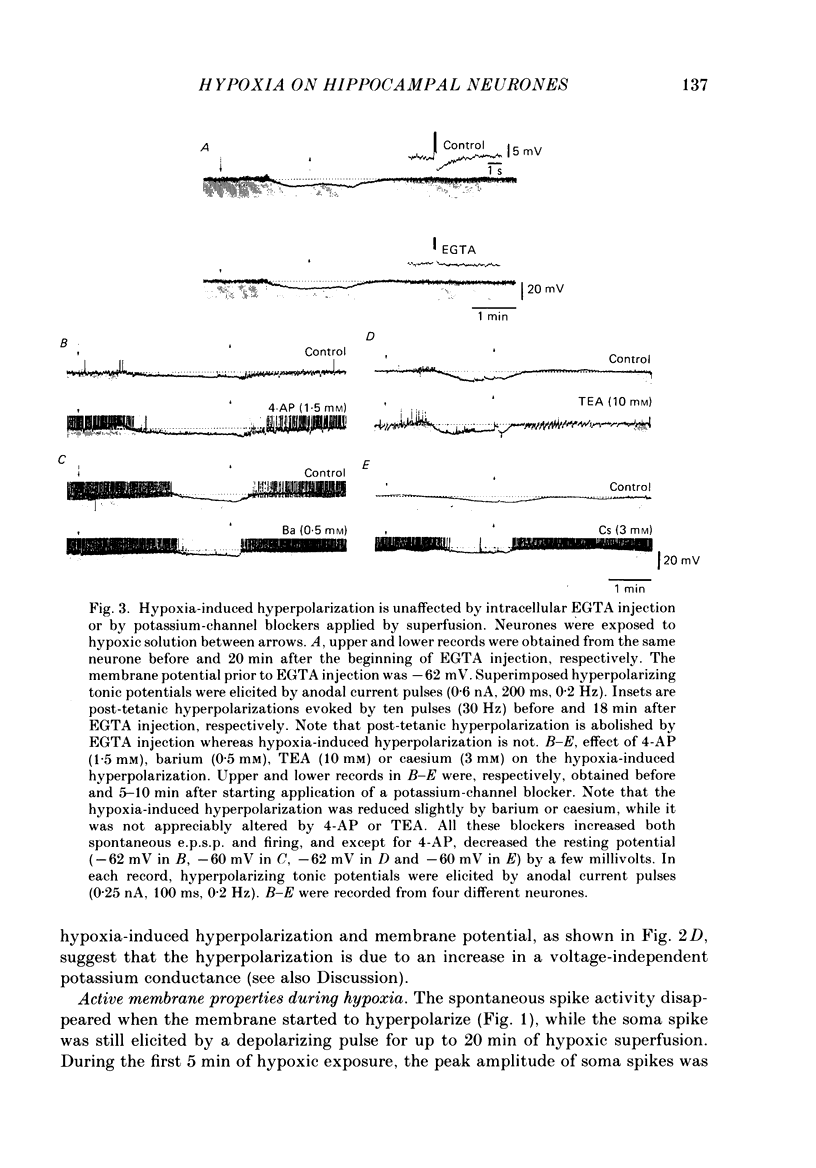








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980 Oct 27;200(1):195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- Buckle P. J., Haas H. L. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982 May;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. H., Fishman R. A. Transient formation of superoxide radicals in polyunsaturated fatty acid-induced brain swelling. J Neurochem. 1980 Oct;35(4):1004–1007. doi: 10.1111/j.1471-4159.1980.tb07100.x. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Drouin H., The R. The effect of reducing extracellular pH on the membrane currents of the ranvier node. Pflugers Arch. 1969;313(1):80–88. doi: 10.1007/BF00586331. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Hoffer B. J. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980 May;69(1):59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Baumgärtl H., Lübbers D. W. Limiting section thickness of guinea pig olfactory cortical slices studied from tissue pO2 values and electrical activities. Pflugers Arch. 1982 Mar;393(1):83–87. doi: 10.1007/BF00582396. [DOI] [PubMed] [Google Scholar]
- Ganfield R. A., Nair P., Whalen W. J. Mass transfer, storage, and utilization of O2 in cat cerebral cortex. Am J Physiol. 1970 Sep;219(3):814–821. doi: 10.1152/ajplegacy.1970.219.3.814. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Extracellular potassium concentration in juvenile and adult rat brain cortex during anoxia. Acta Physiol Scand. 1977 Apr;99(4):412–420. doi: 10.1111/j.1748-1716.1977.tb10394.x. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Hounsgaard J., Jahnsen H. Anoxia increases potassium conductance in hippocampal nerve cells. Acta Physiol Scand. 1982 Jul;115(3):301–310. doi: 10.1111/j.1748-1716.1982.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984 Jul;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass I. S., Lipton P. Mechanisms involved in irreversible anoxic damage to the in vitro rat hippocampal slice. J Physiol. 1982 Nov;332:459–472. doi: 10.1113/jphysiol.1982.sp014424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P., Whittingham T. S. Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea-pig hippocampus. J Physiol. 1982 Apr;325:51–65. doi: 10.1113/jphysiol.1982.sp014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P., Whittingham T. S. The effect of hypoxia on evoked potentials in the in vitro hippocampus. J Physiol. 1979 Feb;287:427–438. doi: 10.1113/jphysiol.1979.sp012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., Prelevic S., Santini M. Hyperpolarizing increase in membrane conductance in hippocampal neurons. Brain Res. 1968 Feb;7(2):310–312. doi: 10.1016/0006-8993(68)90109-1. [DOI] [PubMed] [Google Scholar]
- Reid K. H., Schurr A., Tseng M. T., Edmonds H. L., Jr Resistance to hypoxia in the rat hippocampal slice. Brain Res. 1984 Jun 8;302(2):387–391. doi: 10.1016/0006-8993(84)90255-5. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. Synaptic activity mediates death of hypoxic neurons. Science. 1983 Apr 29;220(4596):536–537. doi: 10.1126/science.6836300. [DOI] [PubMed] [Google Scholar]
- Schmidt M. J., Schmidt D. E., Robison G. A. Cyclic adenosine monophosphate in brain areas: microwave irradiation as a means of tissue fixation. Science. 1971 Sep 17;173(4002):1142–1143. doi: 10.1126/science.173.4002.1142. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Electrical properties of neurones in the olfactory cortex slice in vitro. J Physiol. 1978 Feb;275:535–546. doi: 10.1113/jphysiol.1978.sp012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980 Oct;44(4):827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984 Jun;51(6):1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res. 1974 May 31;72(1):79–97. doi: 10.1016/0006-8993(74)90652-0. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of norepinephrine in the rat hippocampus: intracellular studies in the slice preparation. Brain Res. 1981 Feb 9;206(1):107–128. doi: 10.1016/0006-8993(81)90104-9. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1(2):155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- Whittingham T. S., Lust W. D., Passonneau J. V. An in vitro model of ischemia: metabolic and electrical alterations in the hippocampal slice. J Neurosci. 1984 Mar;4(3):793–802. doi: 10.1523/JNEUROSCI.04-03-00793.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn H. R., Rubio R., Berne R. M. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981 Aug;241(2):H235–H242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]



