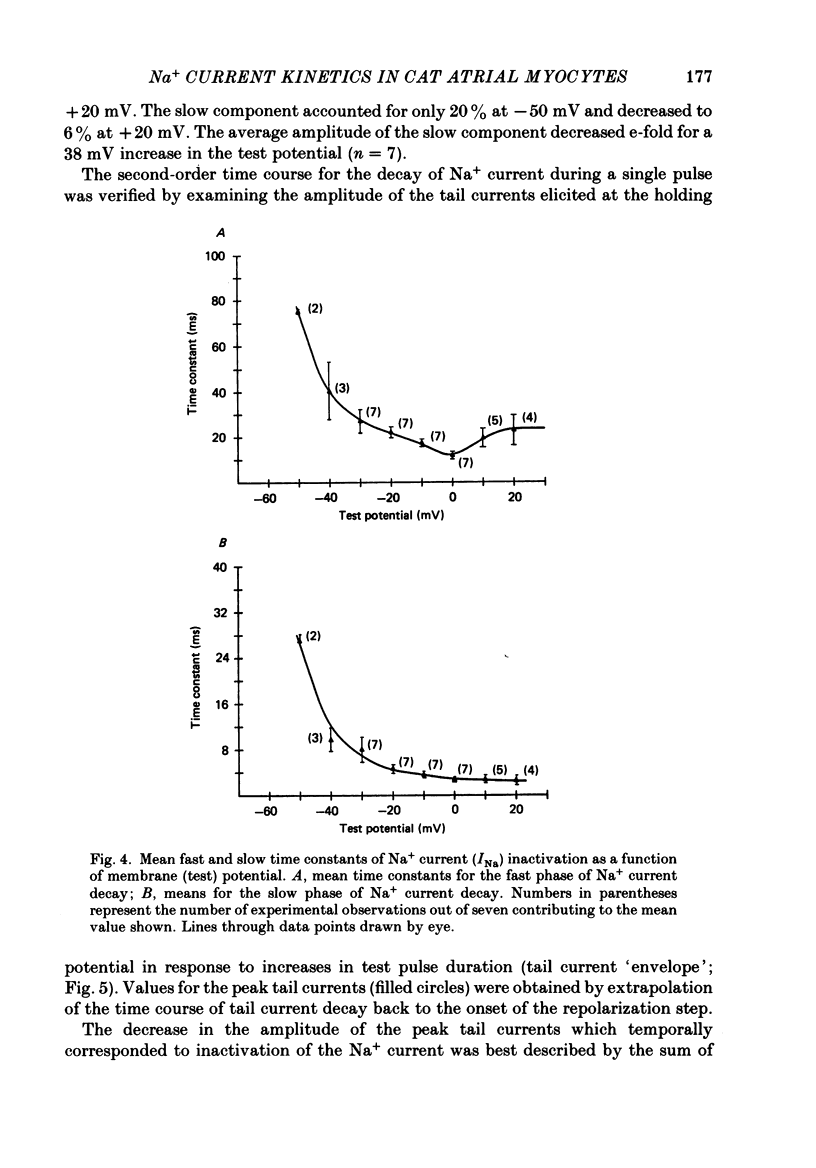
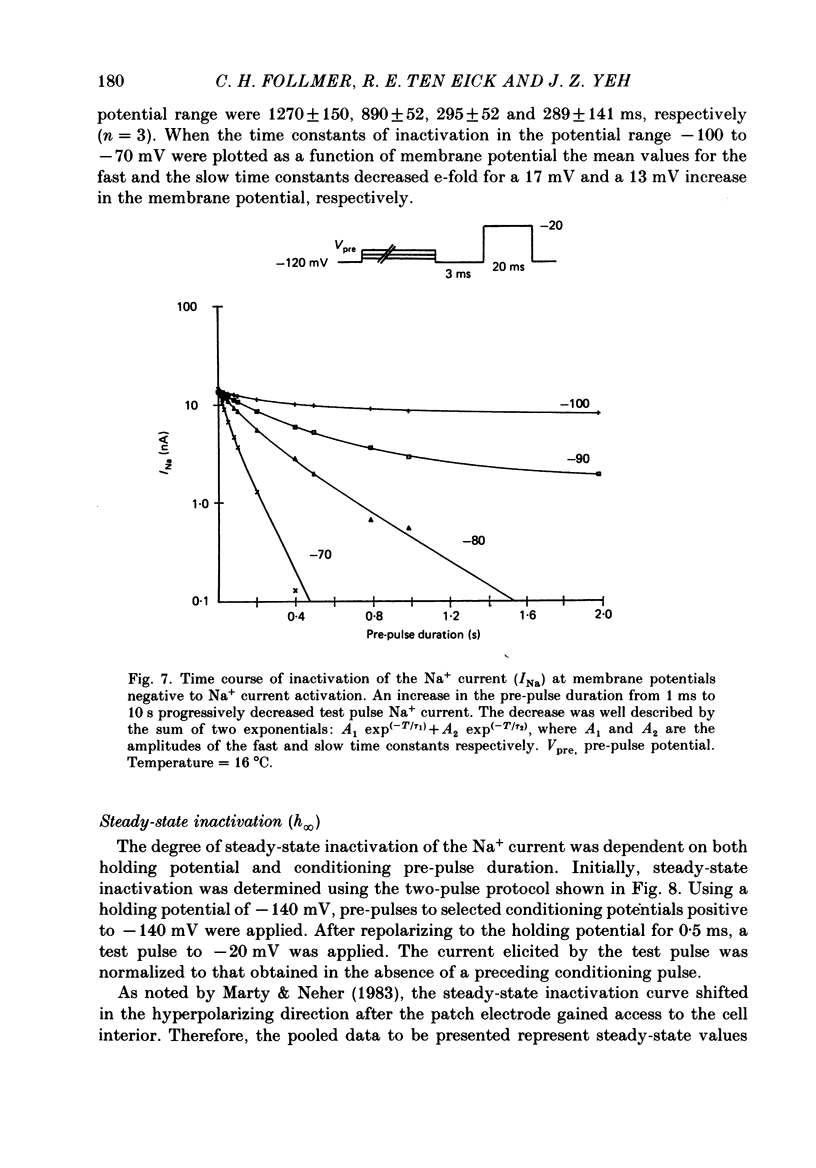
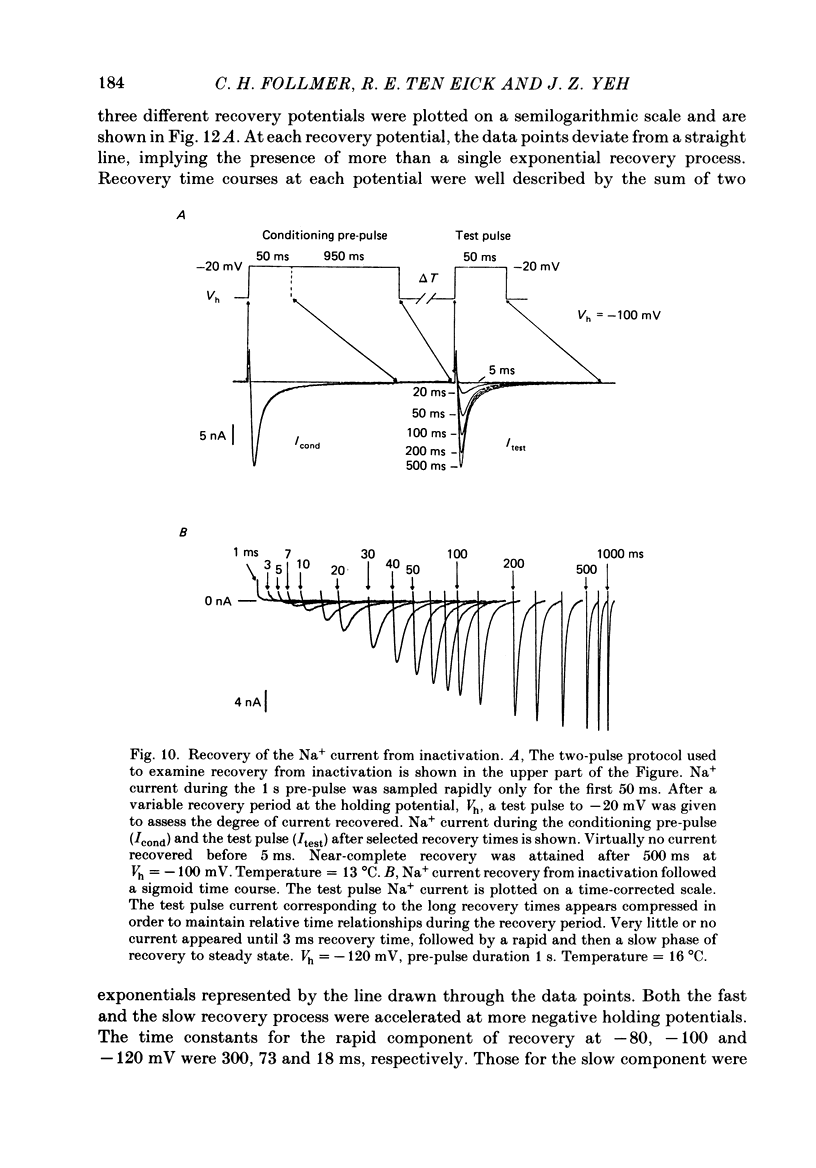
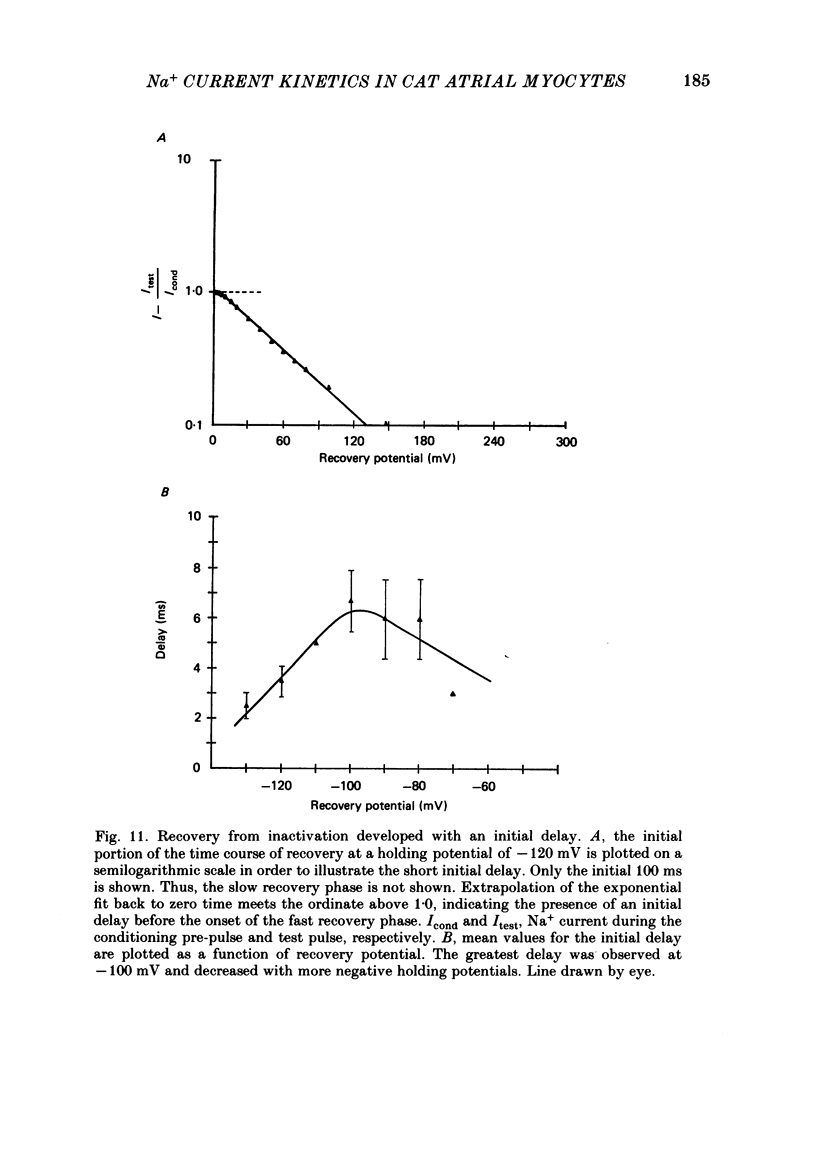
Abstract
1. Na+ current kinetics were studied in isolated atrial myocytes from the adult cat using the single suction-pipette voltage-clamp technique. 2. Current-voltage and conductance-voltage relationships were similar to those described in other cardiac myocyte preparations. 3. Analysis of Na+ current decay using single-pulse, double-pulse and tail current measurements were in agreement and demonstrate a second-order process of current decay. 4. Voltage dependence of steady-state inactivation curves was not symmetrical, having an inflexion at about -90 mV. These results suggest more than a single inactivation process for Na+ channel in the negative potential region. 5. Recovery of Na+ current from inactivation had a sigmoid time course: an initial slow component (delay) followed by a fast and then a second slow component. Increasing the pre-pulse duration slowed the time course of recovery. 6. Taken together, the results were consistent with the presence of multiple inactivated states for the atrial myocyte Na+ channel.
Full text
PDF












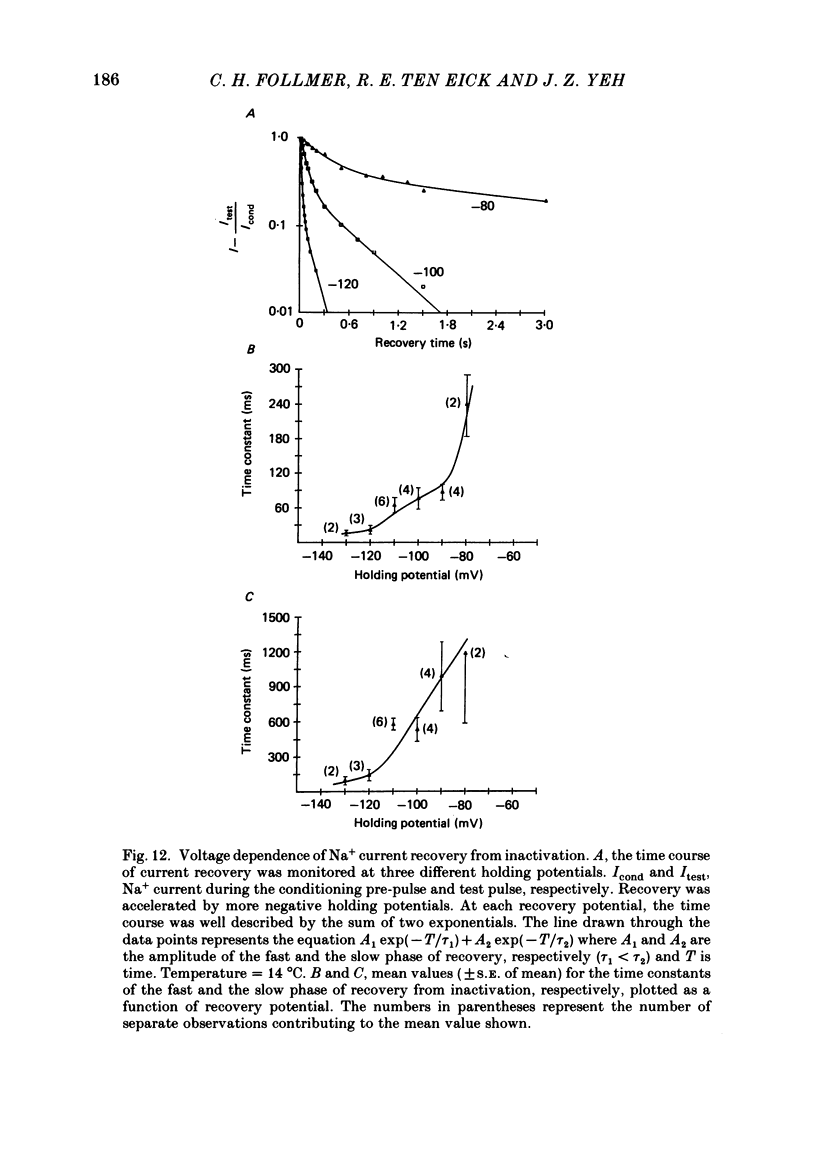
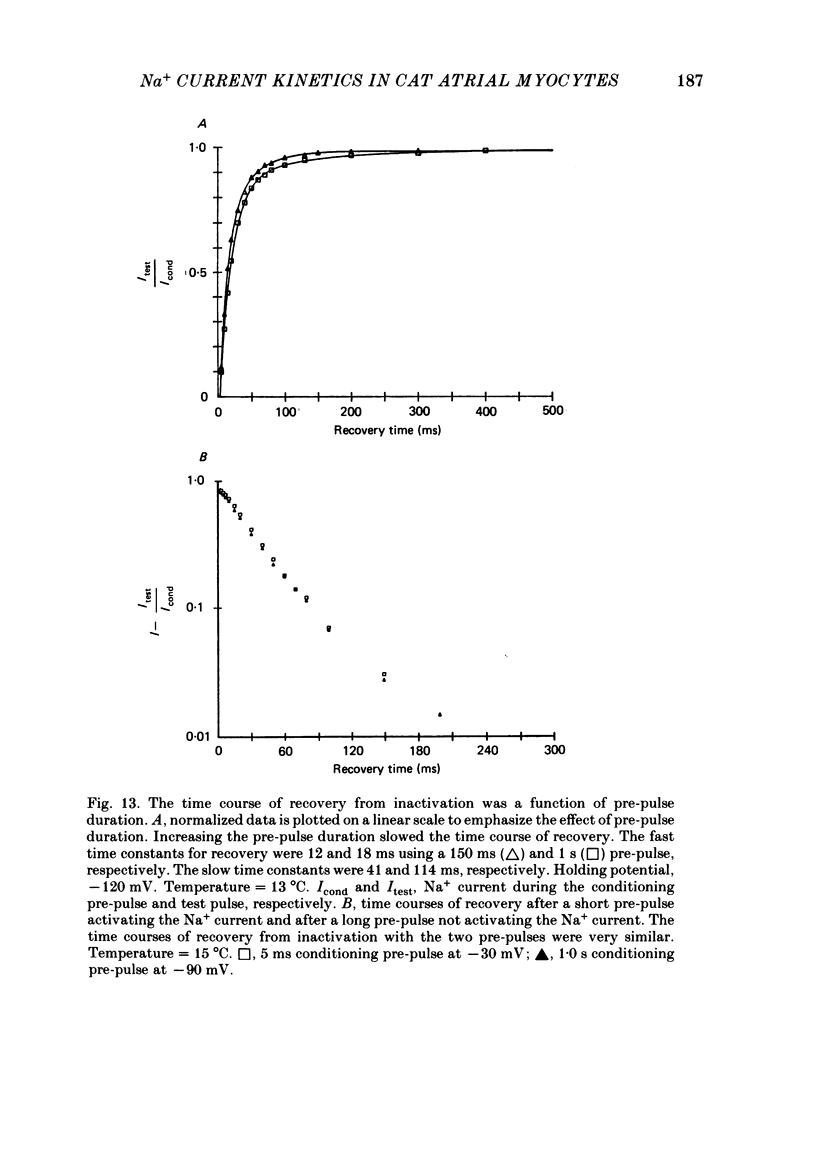

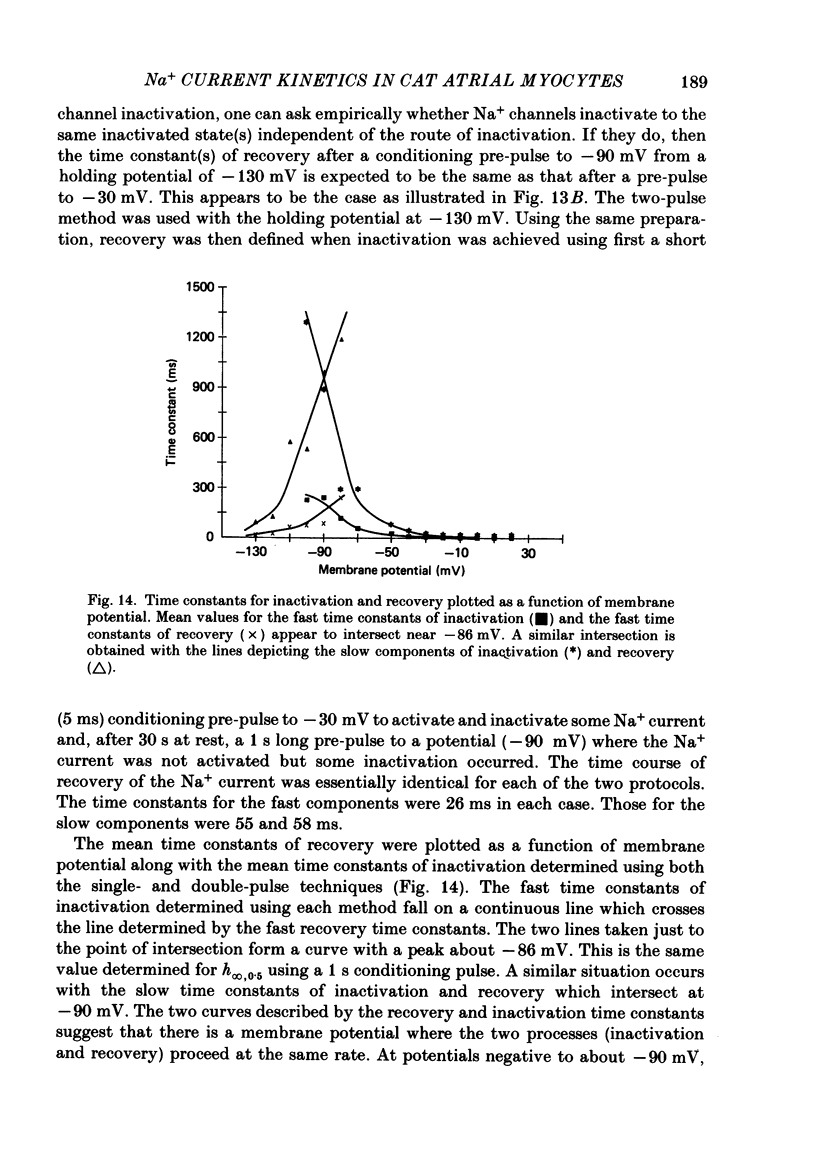








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Inactivation of open and closed sodium channels determined separately. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):147–153. doi: 10.1101/sqb.1983.048.01.017. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewei R., Hering S., Lemke B., Rosenshtraukh L. V., Undrovinas A. I., Wollenberger A. Characterization of the fast sodium current in isolated rat myocardial cells: simulation of the clamped membrane potential. J Physiol. 1982 Apr;325:301–315. doi: 10.1113/jphysiol.1982.sp014151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J. O., McDonald T. F. Sodium currents in segments of human heart cells. Science. 1983 Apr 15;220(4594):320–321. doi: 10.1126/science.6301004. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson C. W., Matsubara T., Hondeghem L. M. Slow inactivation of Vmax in guinea pig ventricular myocardium. Am J Physiol. 1984 Oct;247(4 Pt 2):H645–H654. doi: 10.1152/ajpheart.1984.247.4.H645. [DOI] [PubMed] [Google Scholar]
- Colatsky J. J., Tsien R. W. Sodium channels in rabbit cardiac Purkinje fibres. Nature. 1979 Mar 15;278(5701):265–268. doi: 10.1038/278265a0. [DOI] [PubMed] [Google Scholar]
- Colatsky T. J. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibres. J Physiol. 1980 Aug;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. R., Casnellie J. E., Catterall W. A. Selective phosphorylation of the alpha subunit of the sodium channel by cAMP-dependent protein kinase. J Biol Chem. 1982 Jul 25;257(14):7918–7921. [PubMed] [Google Scholar]
- Ebihara L., Johnson E. A. Fast sodium current in cardiac muscle. A quantitative description. Biophys J. 1980 Nov;32(2):779–790. doi: 10.1016/S0006-3495(80)85016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. A note on the reactivation of the fast sodium current in spherical clusters of embryonic chick heart cells. Biophys J. 1983 May;42(2):191–194. doi: 10.1016/S0006-3495(83)84385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., January C. T., Makielski J. C. New studies of the excitatory sodium currents in heart muscle. Circ Res. 1985 Apr;56(4):475–485. doi: 10.1161/01.res.56.4.475. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F., Strauss H. C. Unitary sodium channels in isolated cardiac myocytes of rabbit. Circ Res. 1983 Dec;53(6):823–829. doi: 10.1161/01.res.53.6.823. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. G., Kern R., Einwächter H. M., Tarr M. Kinetics of Na inactivation in frog atria. Pflugers Arch. 1971;323(2):141–157. doi: 10.1007/BF00586445. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson E., Barr L., Connor J. A. An equivalent circuit for small atrial trabeculae of frog. Biophys J. 1975 Oct;15(10):1069–1085. doi: 10.1016/S0006-3495(75)85883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Fawcett D. W. The ultrastructure of the cat myocardium. II. Atrial muscle. J Cell Biol. 1969 Jul;42(1):46–67. doi: 10.1083/jcb.42.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Moore L. E., Schmid A., Isenberg G. Linear electrical properties of isolated cardiac cells. J Membr Biol. 1984;81(1):29–40. doi: 10.1007/BF01868807. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet M. D. Effect of lidocaine on fast and slow inactivation of sodium current in rat ventricular cells. J Pharmacol Exp Ther. 1982 Oct;223(1):235–240. [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Rudy B. Inactivation in Myxicola giant axons responsible for slow and accumulative adaptation phenomena. J Physiol. 1981 Mar;312:531–549. doi: 10.1113/jphysiol.1981.sp013642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikawa T., Carmeliet E. Slow recovery of the maximal rate of rise (Vmax) of the action potential in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Jul;394(1):90–93. doi: 10.1007/BF01108313. [DOI] [PubMed] [Google Scholar]
- Sanchez-Chapula J., Tsuda Y., Josephson I. R. Voltage- and use-dependent effects of lidocaine on sodium current in rat single ventricular cells. Circ Res. 1983 May;52(5):557–565. doi: 10.1161/01.res.52.5.557. [DOI] [PubMed] [Google Scholar]
- Schauf C. L. Comparison of two-pulse sodium inactivation with reactivation in Myxicola giant axons. Biophys J. 1976 Mar;16(3):245–248. doi: 10.1016/S0006-3495(76)85684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. H., Hemwall E. L., Marino T. A., Houser S. R. Isolation and morphology of calcium-tolerant feline ventricular myocytes. Am J Physiol. 1983 Nov;245(5 Pt 1):H891–H896. doi: 10.1152/ajpheart.1983.245.5.H891. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Waugh R. A. The ultrastructure of the mammalian cardiac muscle cell--with special emphasis on the tubular membrane systems. A review. Am J Pathol. 1976 Jan;82(1):192–232. [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984 Oct;84(4):535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y. I., Timin E. N., Bendukidze Z. A., Burnashev N. A. Patch-voltage-clamp method for measuring fast inward current in single rat heart muscle cells. Pflugers Arch. 1982 Aug;394(2):150–155. doi: 10.1007/BF00582917. [DOI] [PubMed] [Google Scholar]


