Abstract
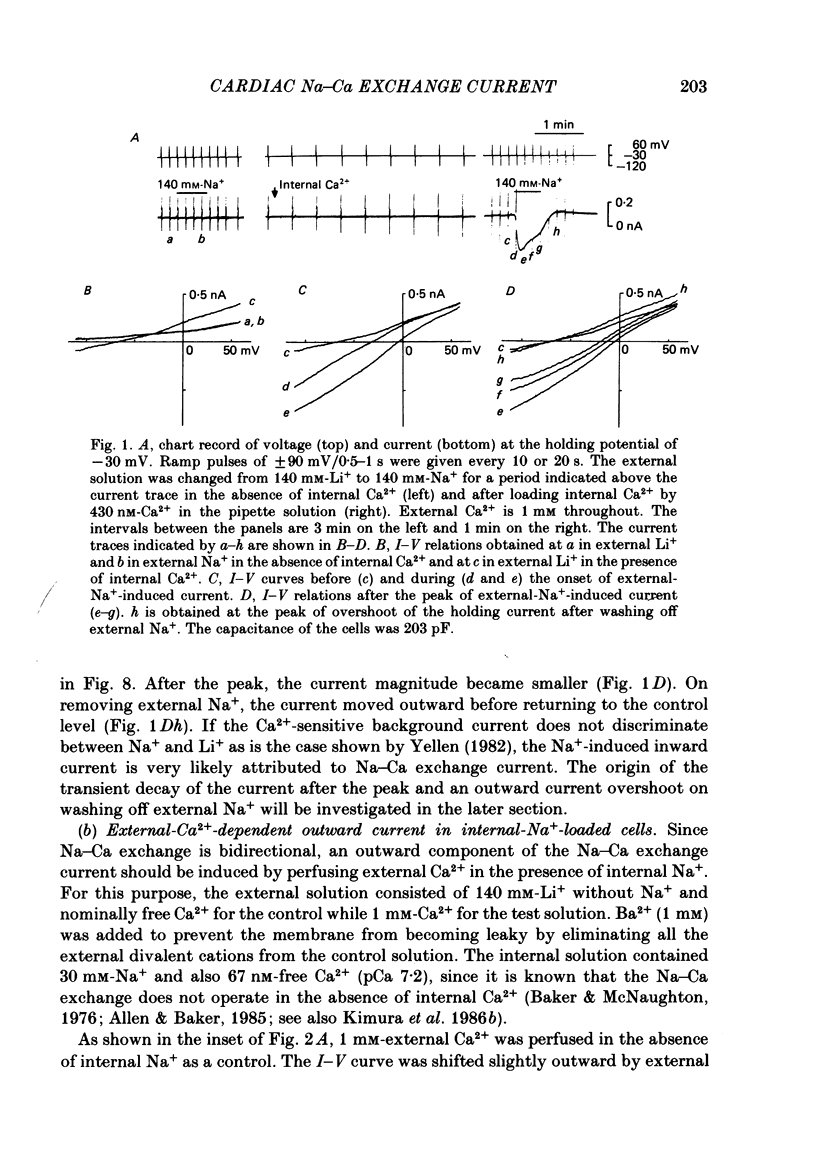
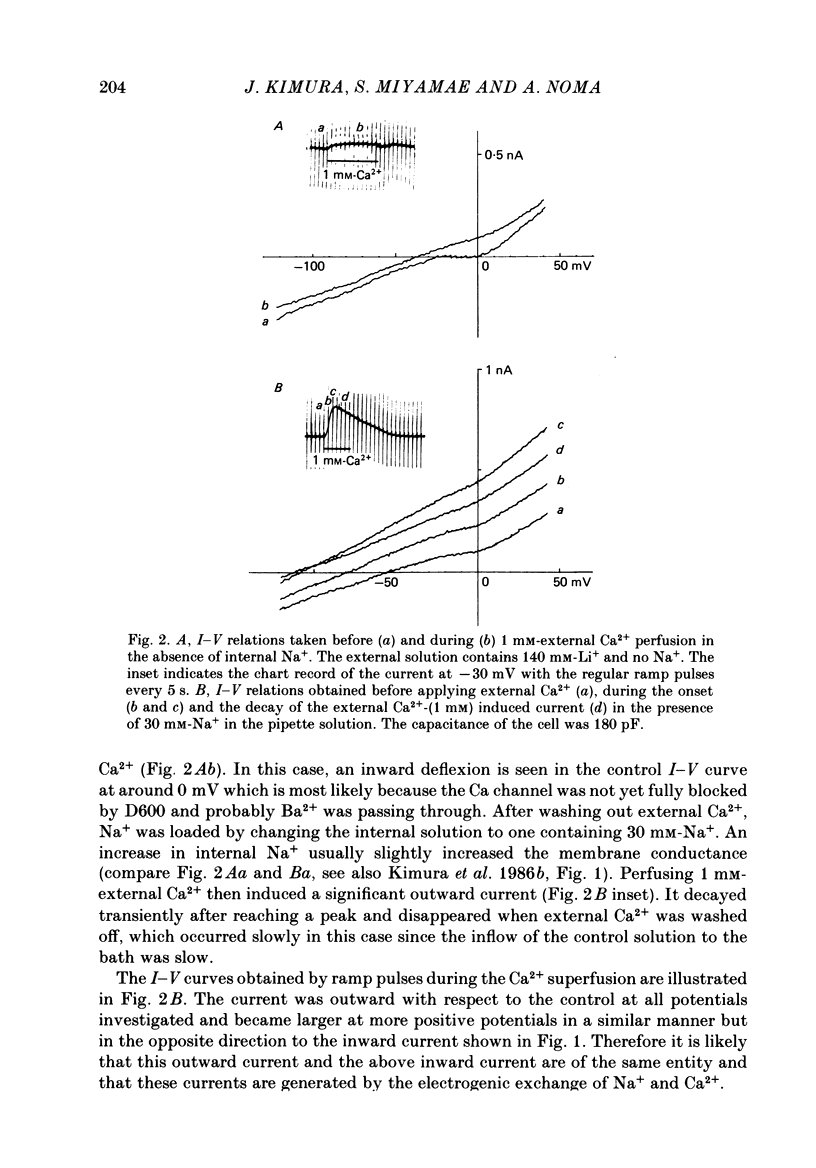
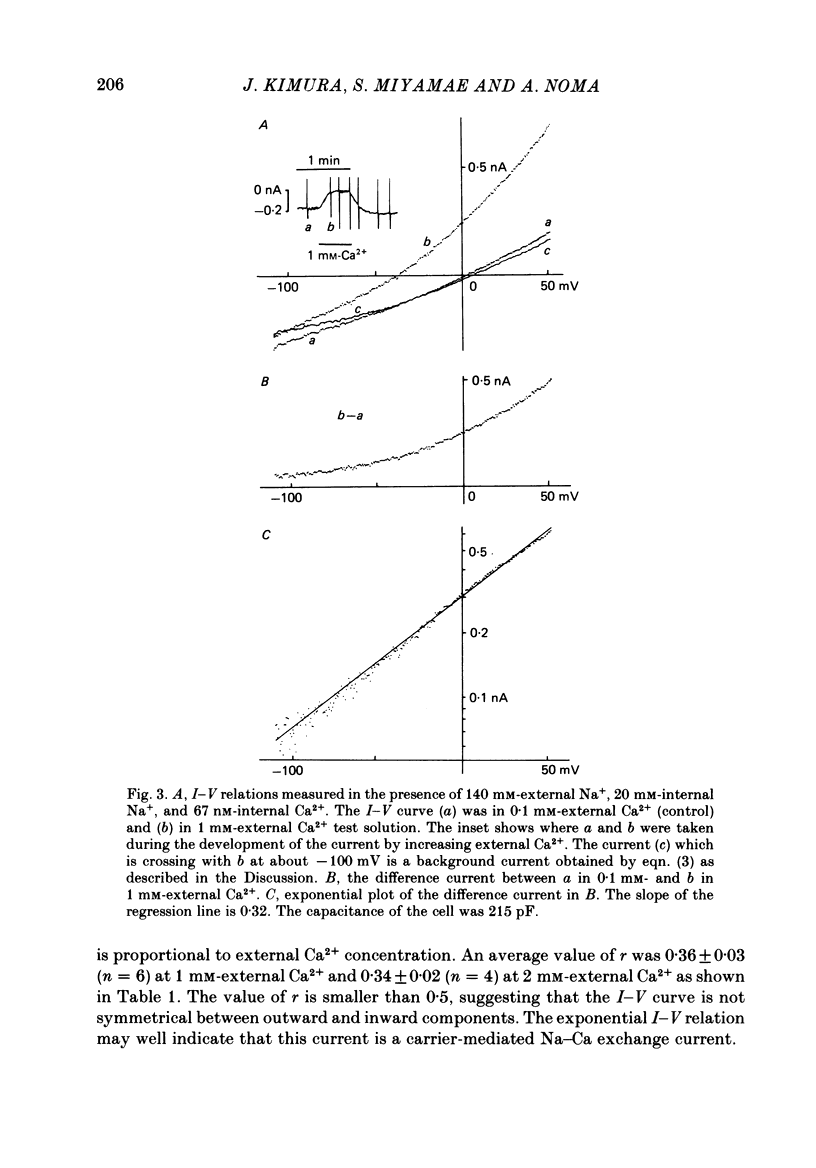
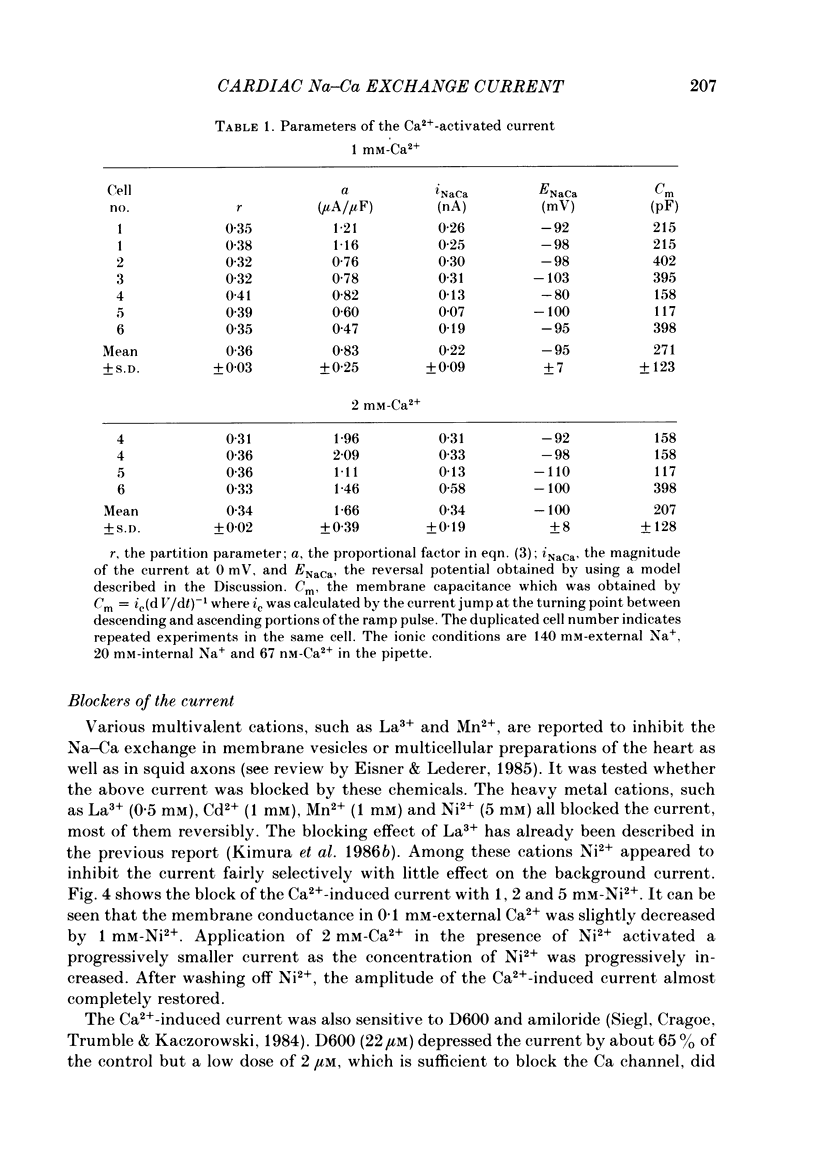
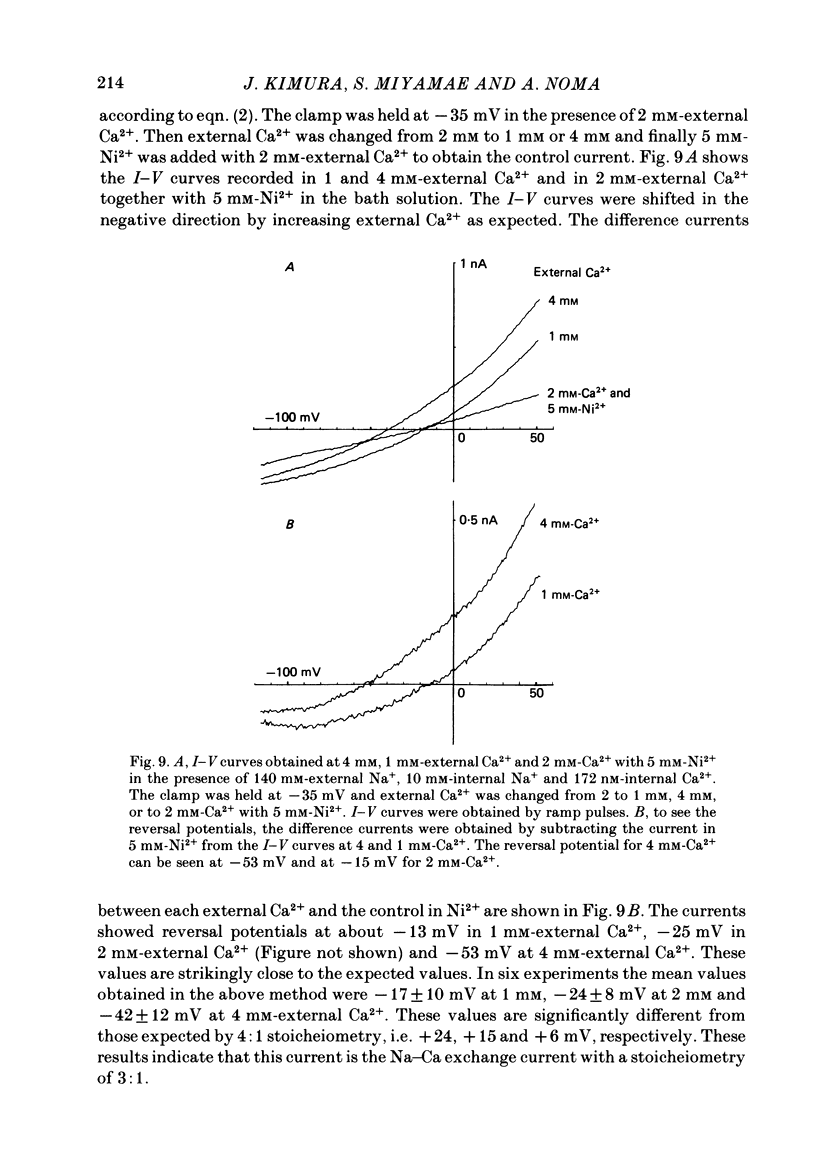
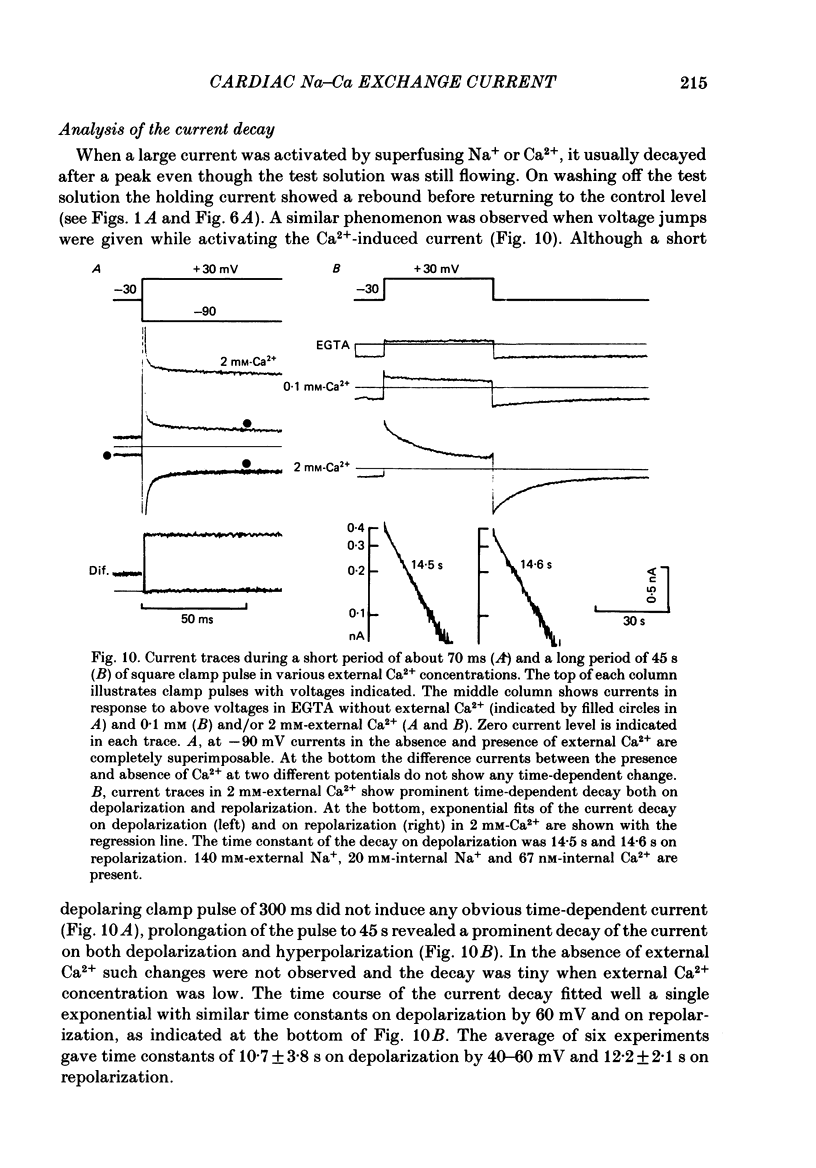
1. The Na-Ca exchange current was investigated in single ventricular cells from guinea-pig hearts by combining the techniques of whole-cell voltage clamp and intracellular perfusion. 2. The membrane conductance was minimized by blocking Ca and K channels as well as the Na-K pump. Under these conditions, when Ca2+ was loaded internally by a pipette solution containing 430 nM-Ca2+, changing the Li+-rich external solution to a Na+-rich one induced a significant inward current. Applying external Na+ in the absence of internal Ca2+ did not appreciably change the current. 3. In contrast, perfusing 1 mM-external Ca2+ in the presence of internal Na+ which was loaded by a 20 mM-Na+ pipette solution, induced a marked outward current. Ca2+ superfusion in the absence of internal Na+ caused only a small current change. 4. The current-voltage relation of external-Ca2+- and external-Na+-induced current showed almost exponential voltage dependence as given by the equation i = a exp (rEF/RT), where a is a scaling factor that determines the magnitude of the current and r is a partition parameter used in the rate theory and represents the position of the energy barrier in the electrical field, which indicates the steepness of the voltage dependence of the current. E, F, R and T have their usual meanings. The value of a was 1-2 microA/microF and r about 0.35 for the Ca2+-induced outward current. At very positive or negative potentials, the current magnitude became smaller than expected from an exponential relation. 5. The current was blocked by heavy metal cations, such as La3+, Cd2+, Mn2+ and Ni2+ and partially blocked by amiloride and D600. 6. The temperature coefficient (Q10) value of the Ca2+-induced outward current was 3.6 +/- 0.4 (n = 4) at 0 mV and 4.0 +/- 0.9 at 50 mV in the range between 21 and 36 degrees C. 7. The outward current magnitude showed a sigmoidal dependence upon the external Ca2+ concentration with a half-maximum concentration, K1/2 of 1.38 mM and a Hill coefficient of 0.9 +/- 0.2 (n = 5). 8. Sr2+ could replace Ca2+ with K1/2 of 7 mM. Mg2+ and Ba2+, however, did not replace Ca2+. 9. The inward current component also showed a sigmoidal external Na+ dependence with K1/2 of 87.5 +/- 10.7 mM and a Hill coefficient of 2.9 +/- 0.4 (n = 6). 10. The reversal potential of the current was obtained near the values expected for 3 Na+:1 Ca2+ exchange.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
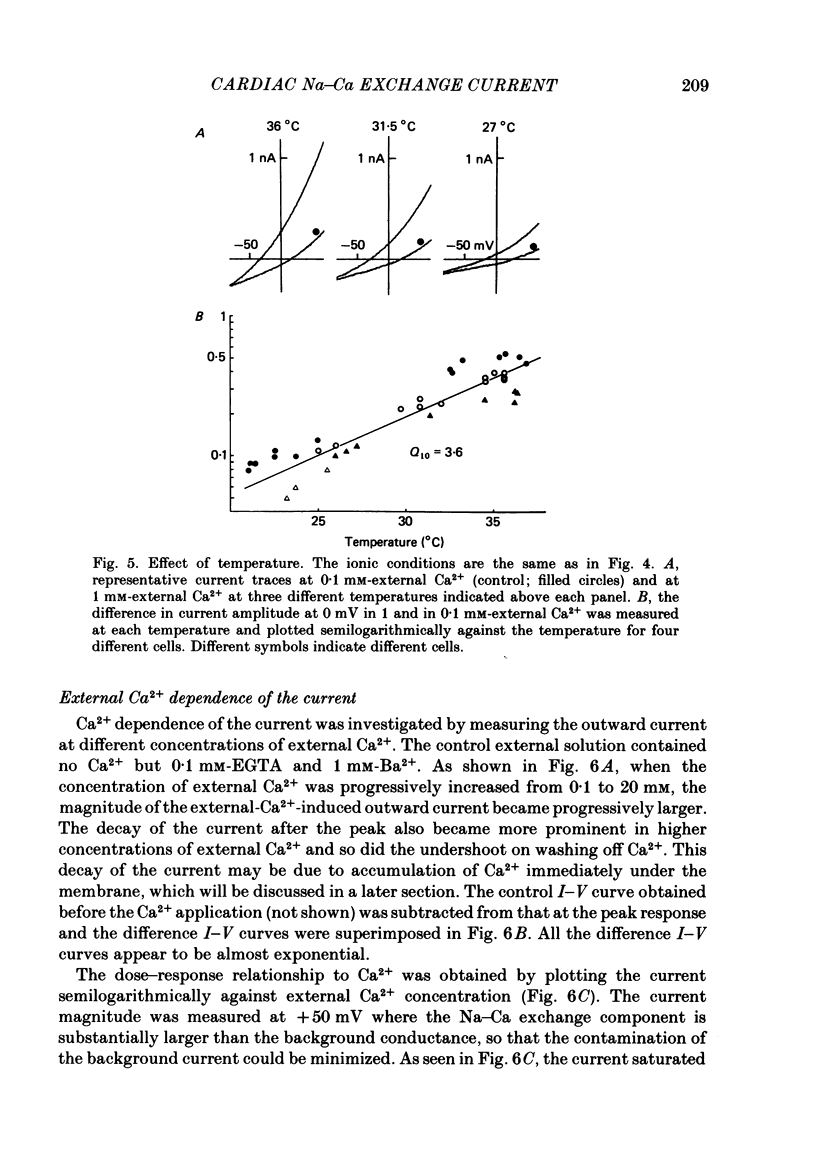
- Allen T. J., Baker P. F. Intracellular Ca indicator Quin-2 inhibits Ca2+ inflow via Na/Ca exchange in squid axon. 1985 Jun 27-Jul 3Nature. 315(6022):755–756. doi: 10.1038/315755a0. [DOI] [PubMed] [Google Scholar]
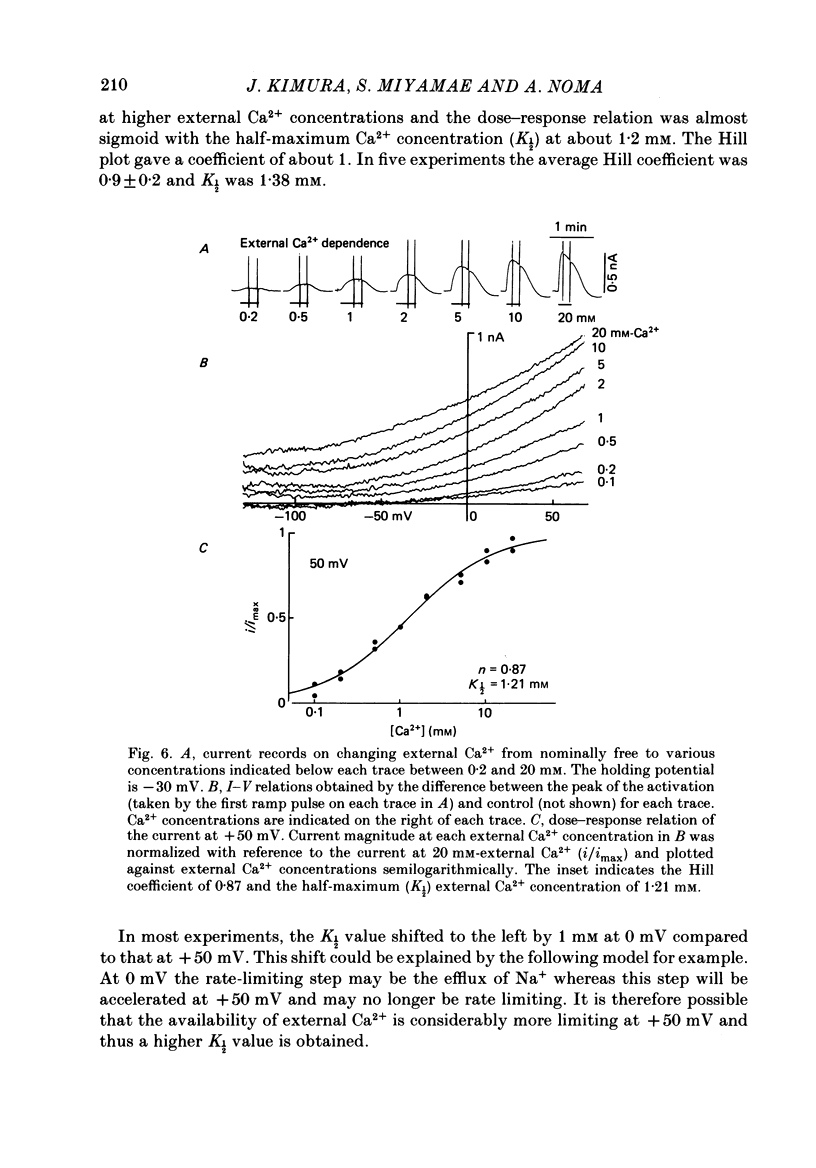
- Arlock P., Katzung B. G. Effects of sodium substitutes on transient inward current and tension in guinea-pig and ferret papillary muscle. J Physiol. 1985 Mar;360:105–120. doi: 10.1113/jphysiol.1985.sp015606. [DOI] [PMC free article] [PubMed] [Google Scholar]
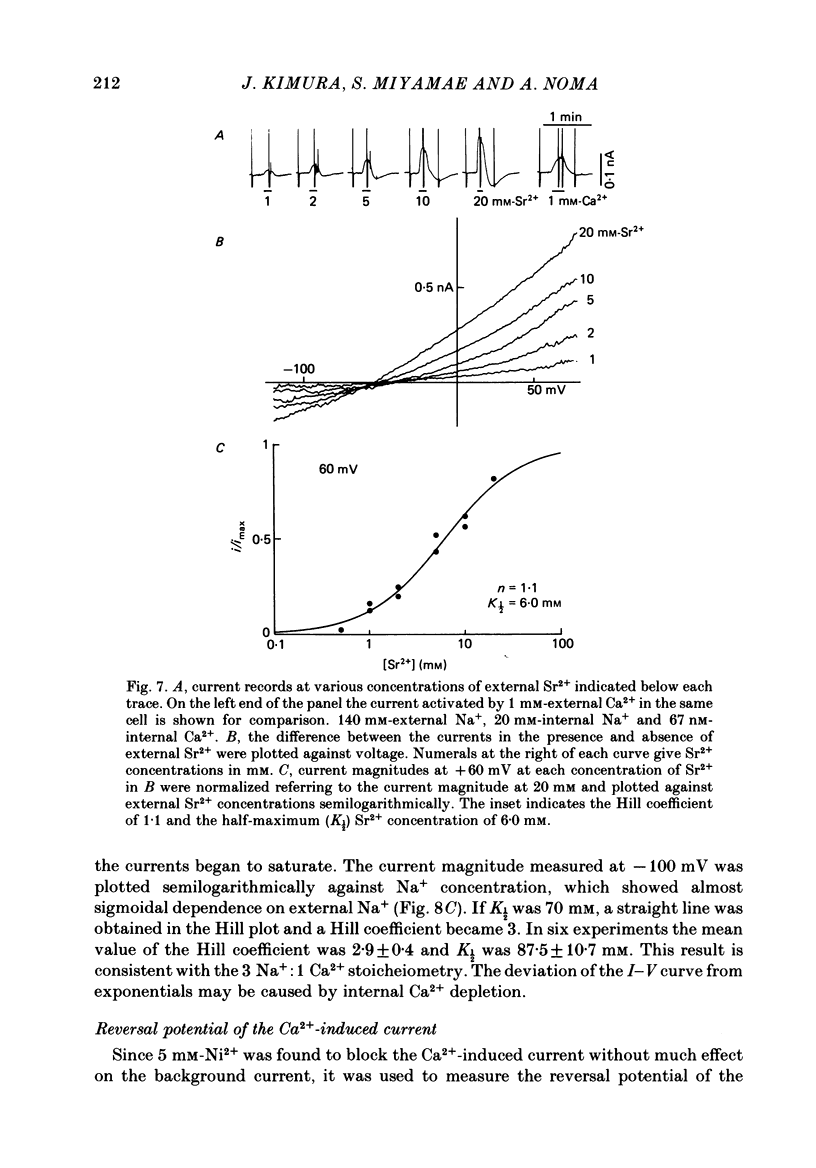
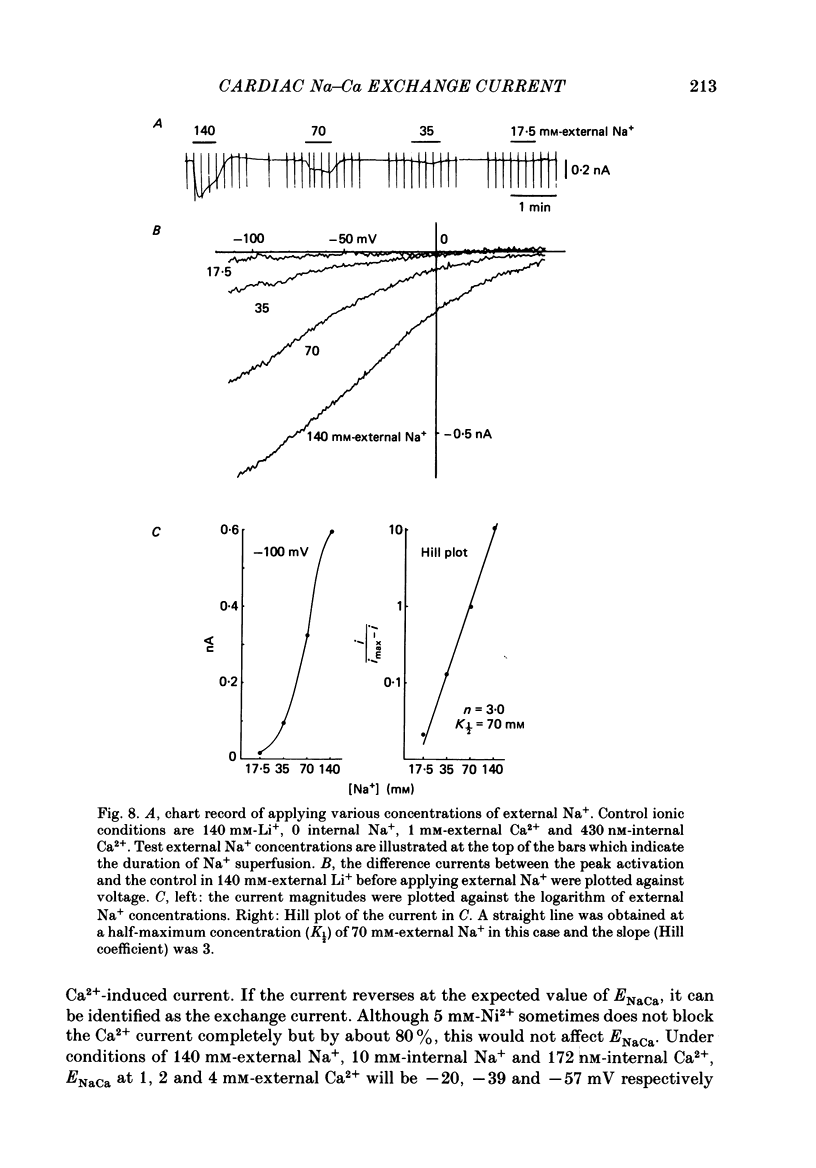
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M., Weer P. Calcium efflux from internally dialyzed squid axons: the influence of external and internal cations. J Supramol Struct. 1974;2(5-6):558–581. doi: 10.1002/jss.400020505. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J. H., Bassingthwaighte J. B. Uphill sodium transport driven by an inward calcium gradient in heart muscle. Science. 1983 Jan 14;219(4581):178–180. doi: 10.1126/science.6849128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Noble D., Noble S. J., Taupignon A. I. Relationship between the transient inward current and slow inward currents in the sino-atrial node of the rabbit. J Physiol. 1986 Jan;370:299–315. doi: 10.1113/jphysiol.1986.sp015936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Croaboeuf E., Gautier P., Giuraudou P. Potential and tension changes induced by sodium removal in dog Purkinje fibres: role of an electrogenic sodium-calcium exchange. J Physiol. 1981 Feb;311:605–622. doi: 10.1113/jphysiol.1981.sp013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. The calcium pump and sodium-calcium exchange in squid axons. Annu Rev Physiol. 1983;45:313–324. doi: 10.1146/annurev.ph.45.030183.001525. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Bezanilla F., Caputo C., Rojas H. Voltage dependence of the Na/Ca exchange in voltage-clamped, dialyzed squid axons. Na-dependent Ca efflux. J Gen Physiol. 1985 Oct;86(4):457–478. doi: 10.1085/jgp.86.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Na-Ca exchange: stoichiometry and electrogenicity. Am J Physiol. 1985 Mar;248(3 Pt 1):C189–C202. doi: 10.1152/ajpcell.1985.248.3.C189. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoma M., Froehlich J., Reeves J., Sutko J. Kinetics of sodium ion induced calcium ion release in calcium ion loaded cardiac sarcolemmal vesicles: determination of initial velocities by stopped-flow spectrophotometry. Biochemistry. 1982 Apr 13;21(8):1914–1918. doi: 10.1021/bi00537a033. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Sodium-calcium exchange in the heart. Annu Rev Physiol. 1982;44:435–449. doi: 10.1146/annurev.ph.44.030182.002251. [DOI] [PubMed] [Google Scholar]
- Ledvora R. F., Hegyvary C. Dependence of Na+-Ca2+ exchange and Ca2+-Ca2+ exchange on monovalent cations. Biochim Biophys Acta. 1983 Mar 23;729(1):123–136. doi: 10.1016/0005-2736(83)90463-7. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Philipson K. D. Sodium-calcium exchange in plasma membrane vesicles. Annu Rev Physiol. 1985;47:561–571. doi: 10.1146/annurev.ph.47.030185.003021. [DOI] [PubMed] [Google Scholar]
- Pitts B. J. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem. 1979 Jul 25;254(14):6232–6235. [PubMed] [Google Scholar]
- Reeves J. P., Hale C. C. The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem. 1984 Jun 25;259(12):7733–7739. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Competitive interactions of sodium and calcium with the sodium-calcium exchange system of cardiac sarcolemmal vesicles. J Biol Chem. 1983 Mar 10;258(5):3178–3182. [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Blaustein M. P. Calcium efflux from barnacle muscle fibers. Dependence on external cations. J Gen Physiol. 1974 Feb;63(2):144–167. doi: 10.1085/jgp.63.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R., Noma A., Kurachi Y., Irisawa H. Effects of intracellular acidification on membrane currents in ventricular cells of the guinea pig. Circ Res. 1985 Oct;57(4):553–561. doi: 10.1161/01.res.57.4.553. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl P. K., Cragoe E. J., Jr, Trumble M. J., Kaczorowski G. J. Inhibition of Na+/Ca2+ exchange in membrane vesicle and papillary muscle preparations from guinea pig heart by analogs of amiloride. Proc Natl Acad Sci U S A. 1984 May;81(10):3238–3242. doi: 10.1073/pnas.81.10.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., St Louis P. J. Passive and active calcium fluxes across plasma membranes. Prog Biophys Mol Biol. 1980;35(3):135–195. doi: 10.1016/0079-6107(80)90005-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Kokubun S., Noma A., Irisawa H. Spontaneously active cells isolated from the sino-atrial and atrio-ventricular nodes of the rabbit heart. Jpn J Physiol. 1981;31(4):547–558. doi: 10.2170/jjphysiol.31.547. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Isenberg G. Intracellular [Ca2+] transients in voltage clamped cardiac Purkinje fibers. Pflugers Arch. 1982 Jan;392(3):284–290. doi: 10.1007/BF00584312. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


