Abstract
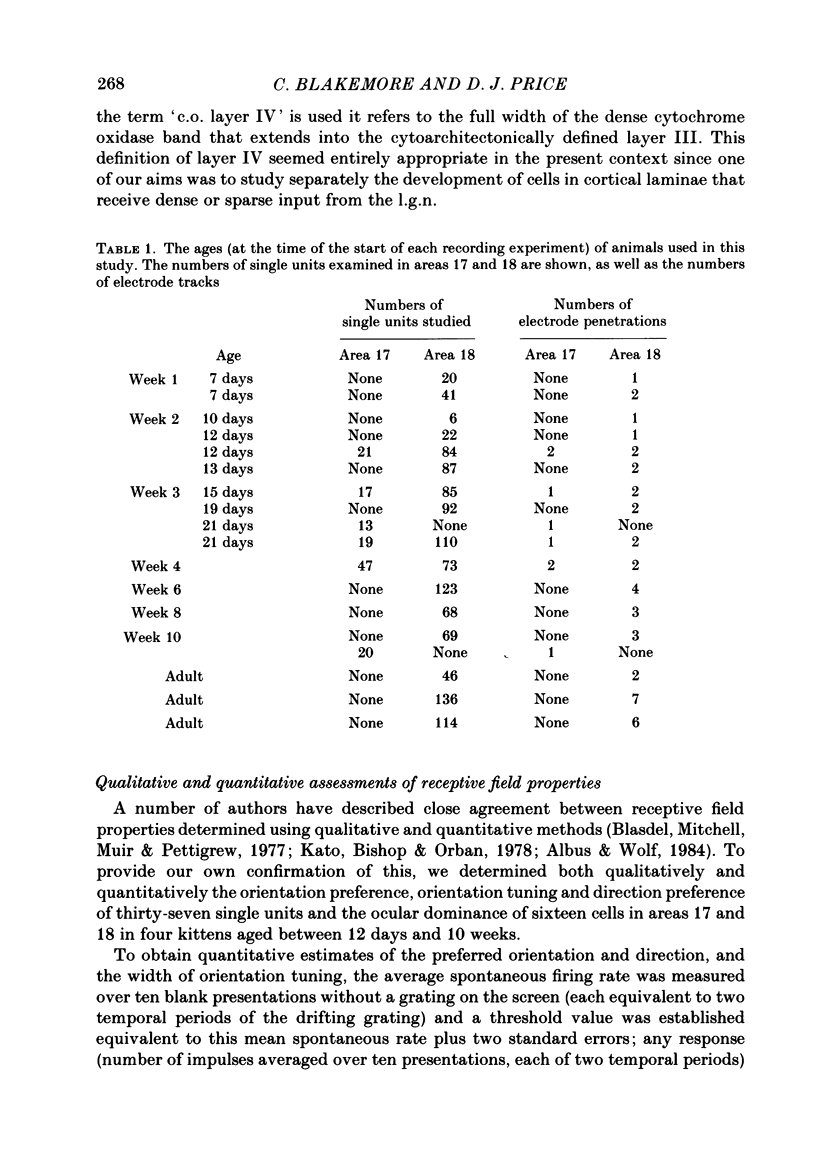
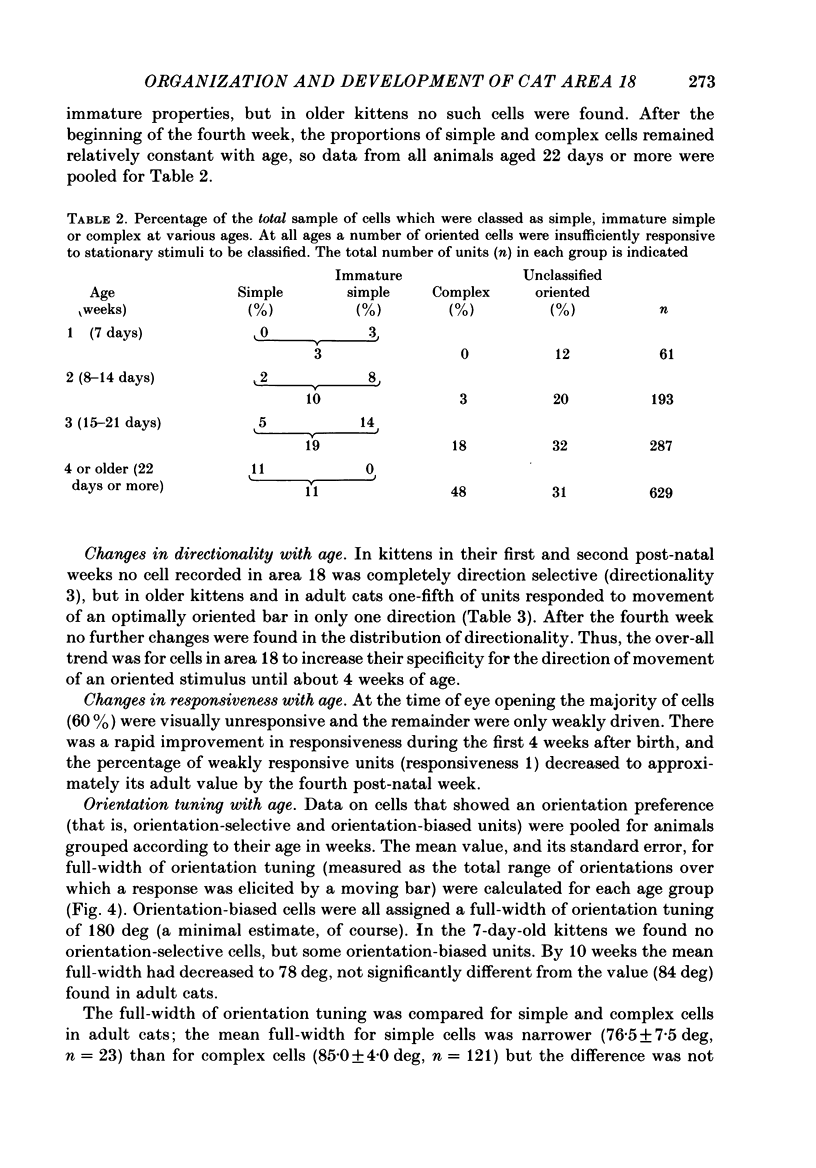
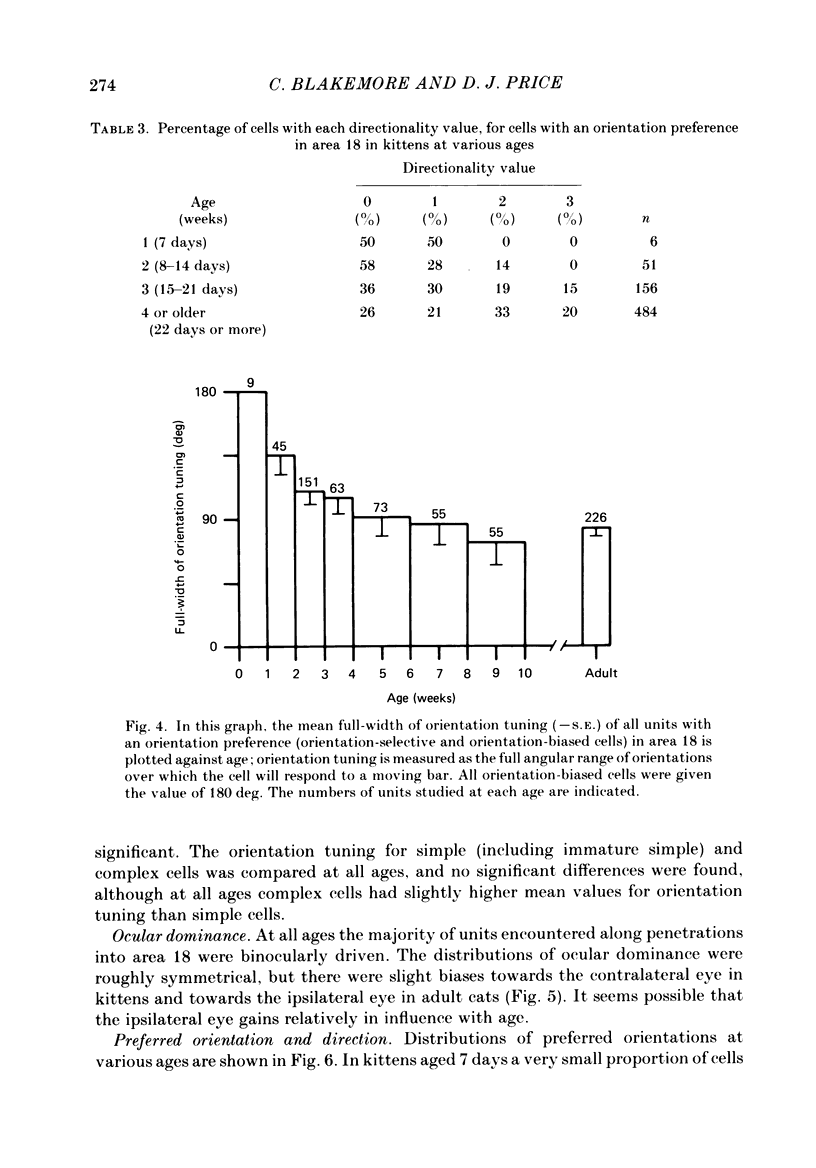
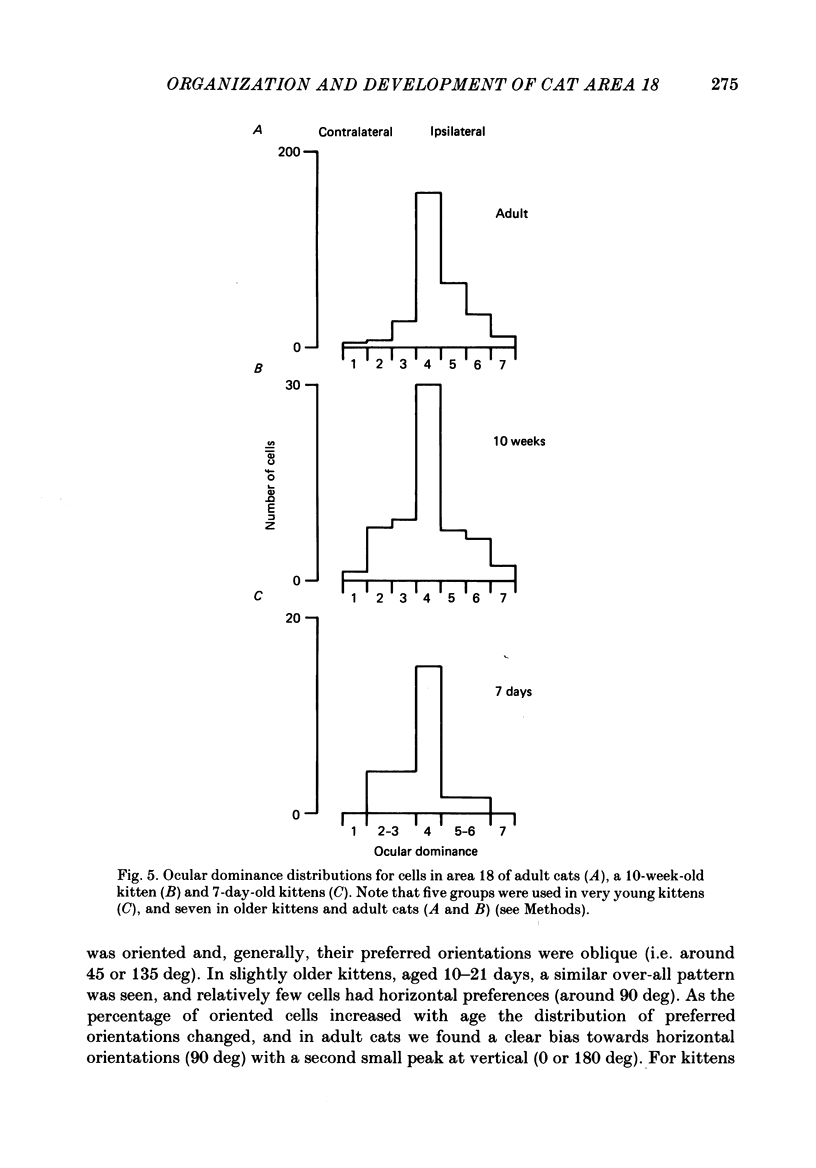
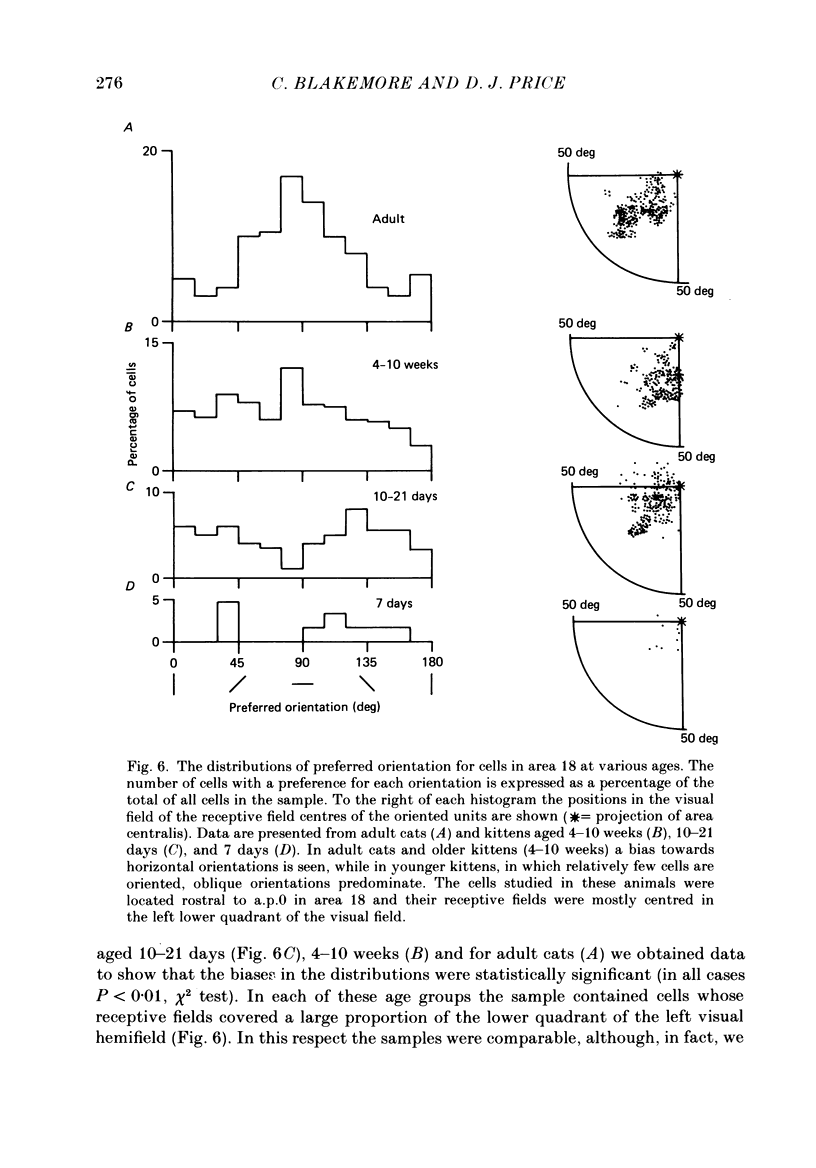
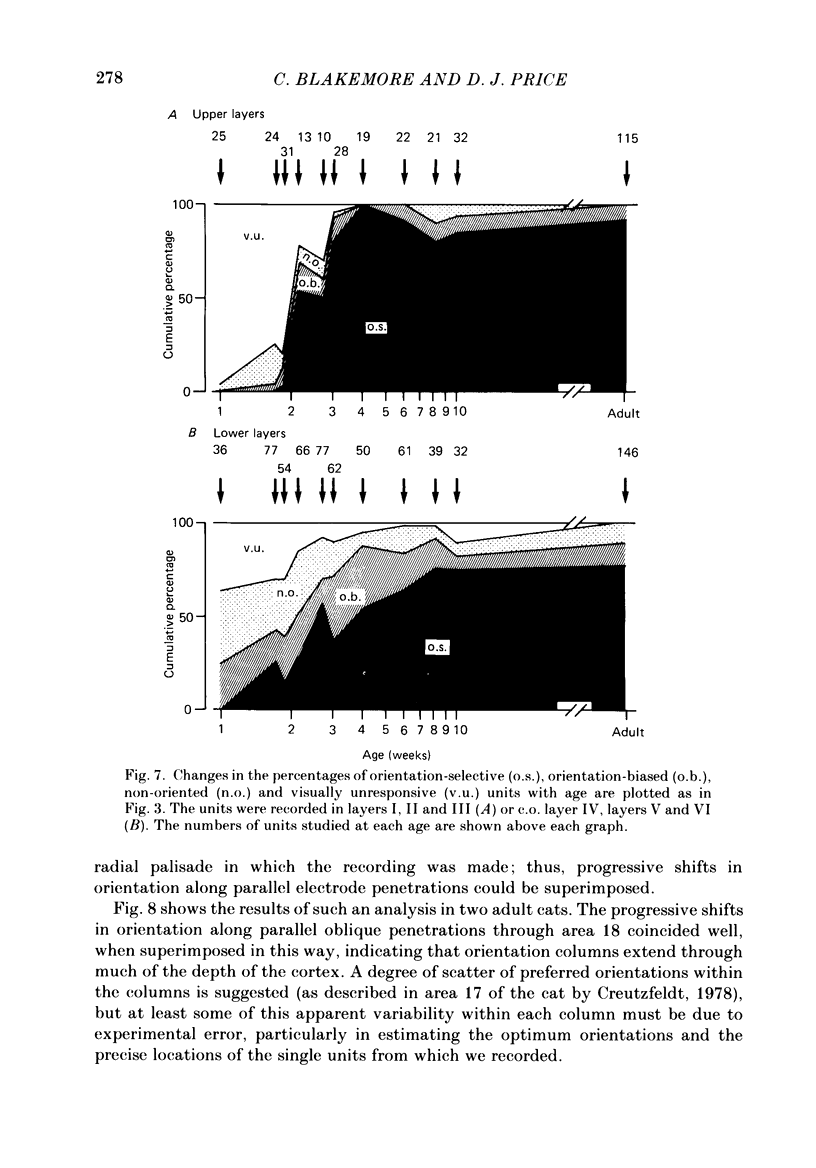
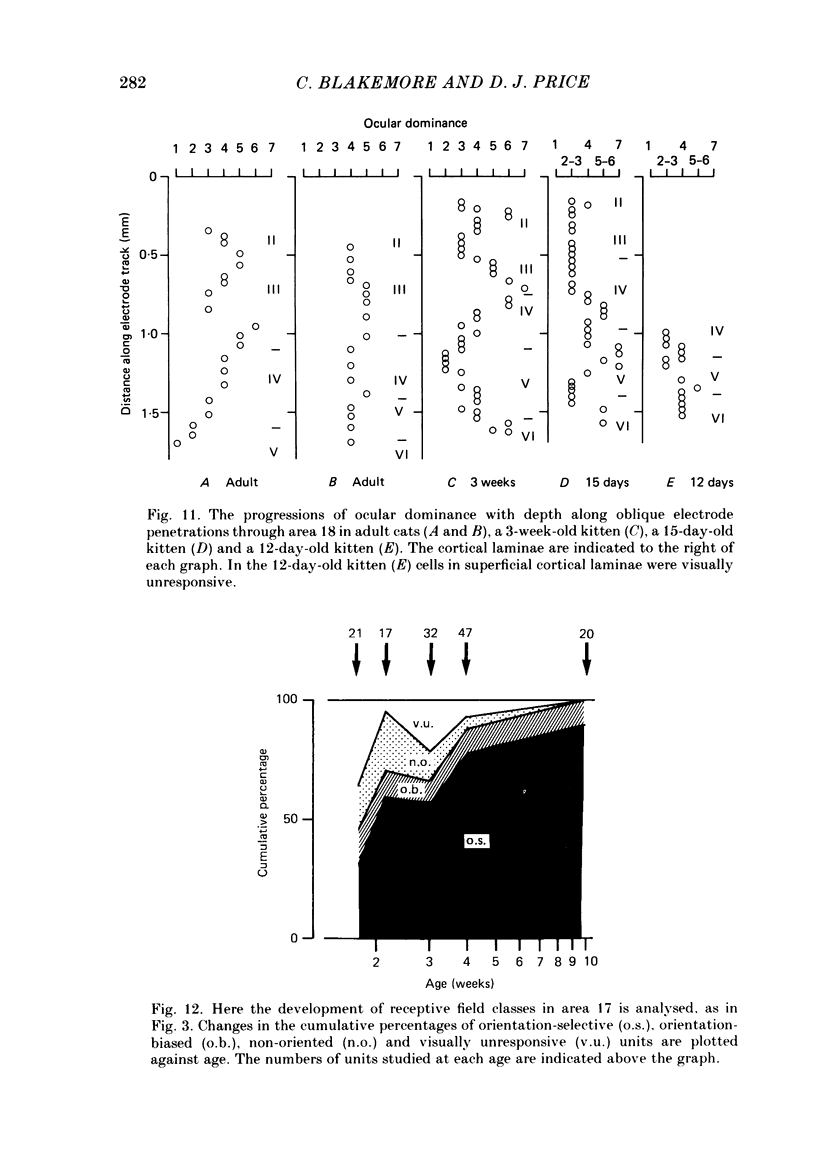
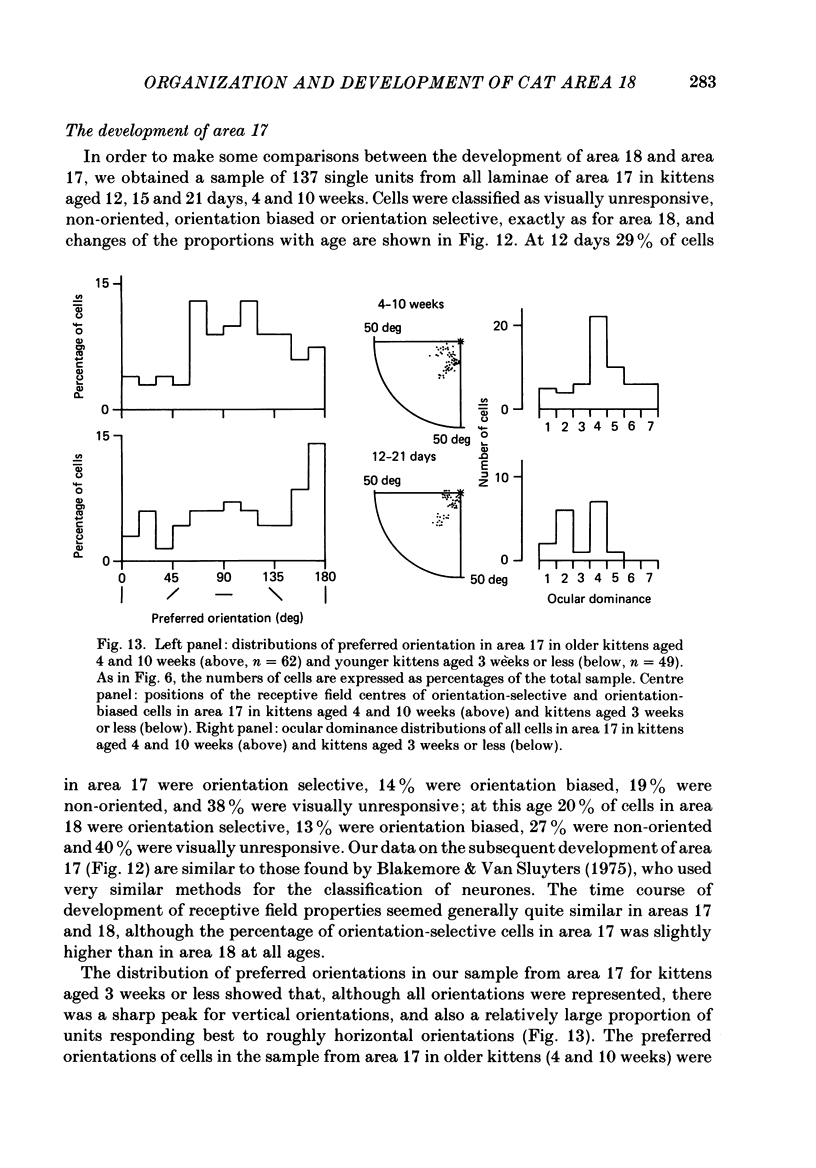
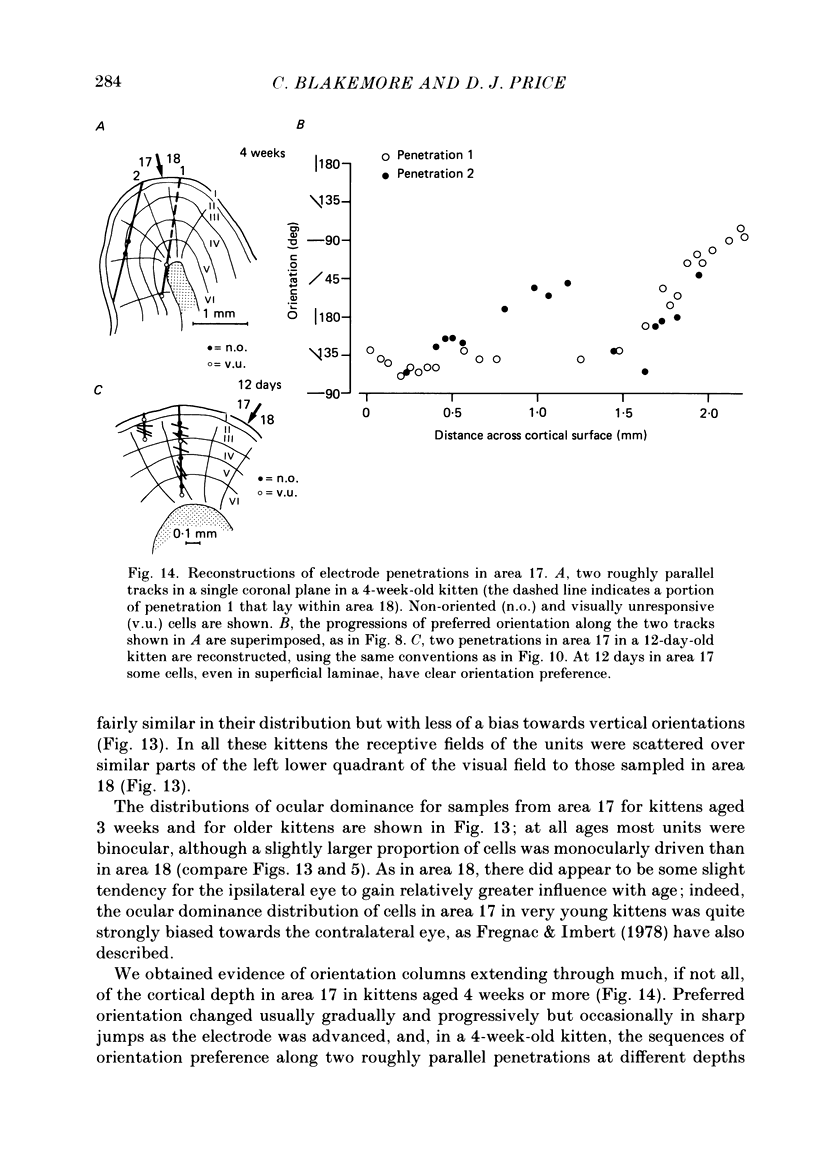
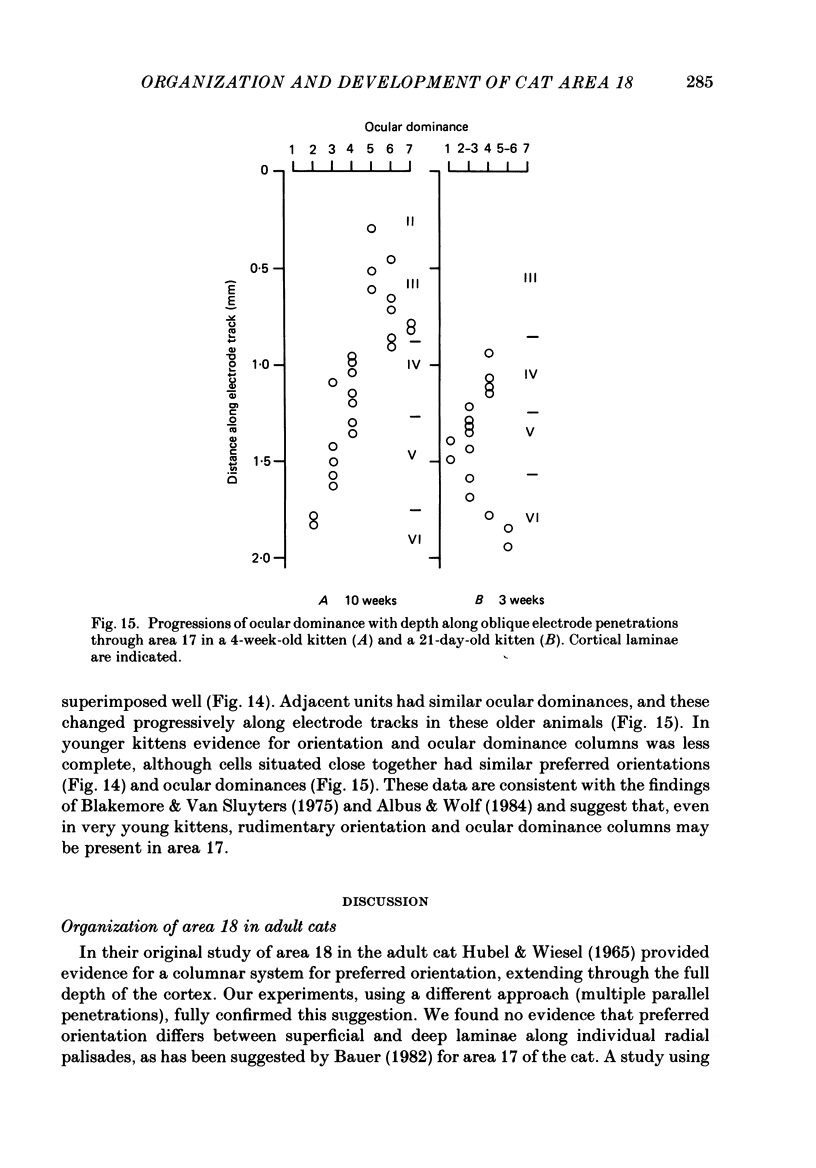
1. We made extracellular recordings from 1176 single units in area 18 of adult cats and kittens aged between 7 days and 10 weeks, and from 137 single units in area 17 in kittens aged between 12 days and 10 weeks. 2. All cells examined in area 18 of adult cats were visually responsive, 84% being orientation selective, 9% orientation biased and 7% non-oriented. Orientation columns and ocular dominance columns were identified. There was an over-all bias towards horizontal and vertical in the distribution of preferred orientations in the rostral part of area 18, where we were recording. 3. In area 18 of 7-day-old, visually inexperienced kittens the majority of cells (60%) were visually unresponsive; the remainder were either non-oriented (25%) or orientation biased (15%), and no orientation-selective units were found. Nevertheless there seemed to be a rudimentary columnar system, even in the youngest animals, in that orientation-biased cells tended to occur in clusters with neighboring neurones having similar orientation preferences. In normal animals of 3 weeks and younger we found that the distribution of preferred orientations of a sample of neurones in area 18 with receptive fields scattered over much of the left lower quadrant of the visual hemifield was biased to oblique orientations. 4. As in adult cats, most cells in area 18 in young kittens were binocularly driven, and periodic alternation of dominance along oblique penetrations, characteristic of ocular dominance columns, was sometimes seen, even at the earliest ages. 5. Many of the developmental changes that we observed in area 18 occurred during the first 4 post-natal weeks. Orientation selectivity, orientation tuning, directionality and responsiveness of neurones matured rapidly over this period. The proportions of simple and complex cells were similar in kittens aged 4 weeks or more to those in adult cats, whereas prior to this most neurones that displayed an orientation preference appeared to be immature simple cells. 6. A laminar analysis revealed that very few units in the superficial layers (I, II and III) in area 18 are visually responsive in kittens during their first and second weeks, but orientation selectivity rapidly develops during the third week. By contrast, even in very young, visually inexperienced kittens, the majority of neurones found in deeper laminae (IV, V and VI) are visually responsive and a few of them already show an orientation preference; however, the subsequent appearance of larger proportions of orientation-selective cells in these lower layers is a more prolonged process than in upper laminae.(ABSTRACT TRUNCATED AT 400 WORDS)
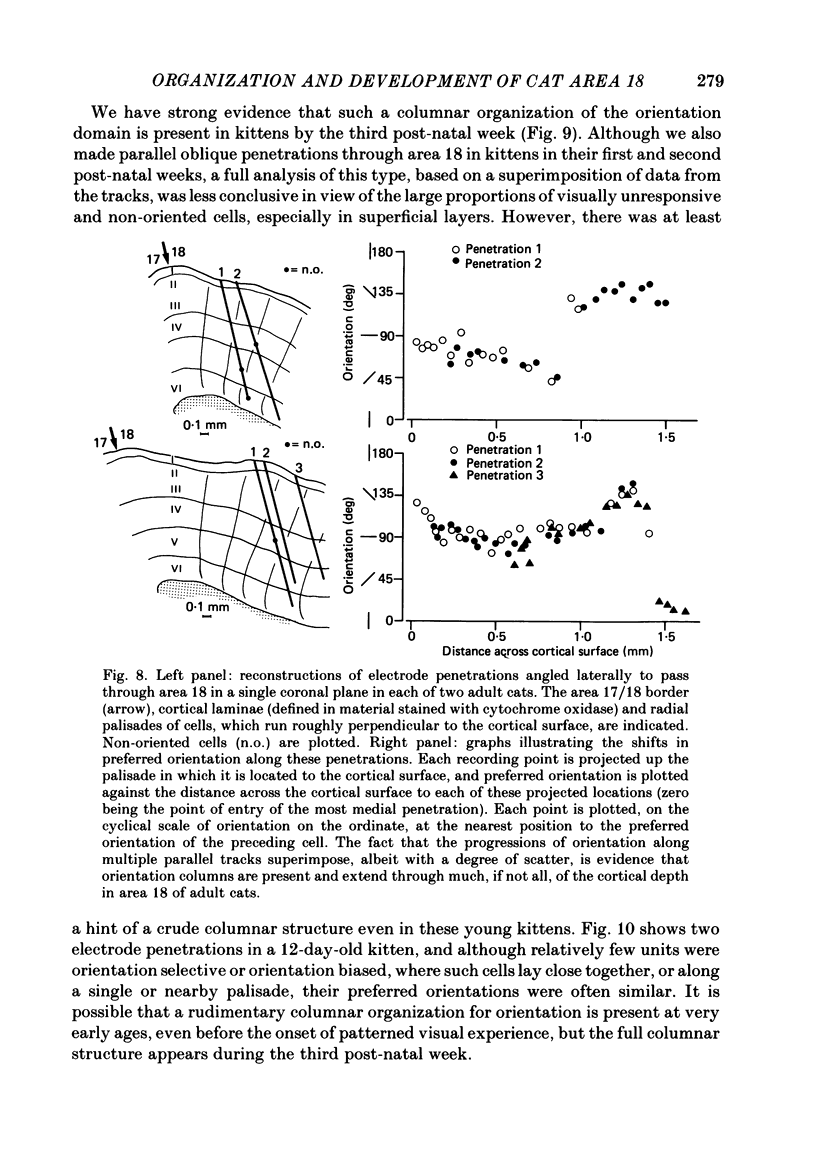
Full text
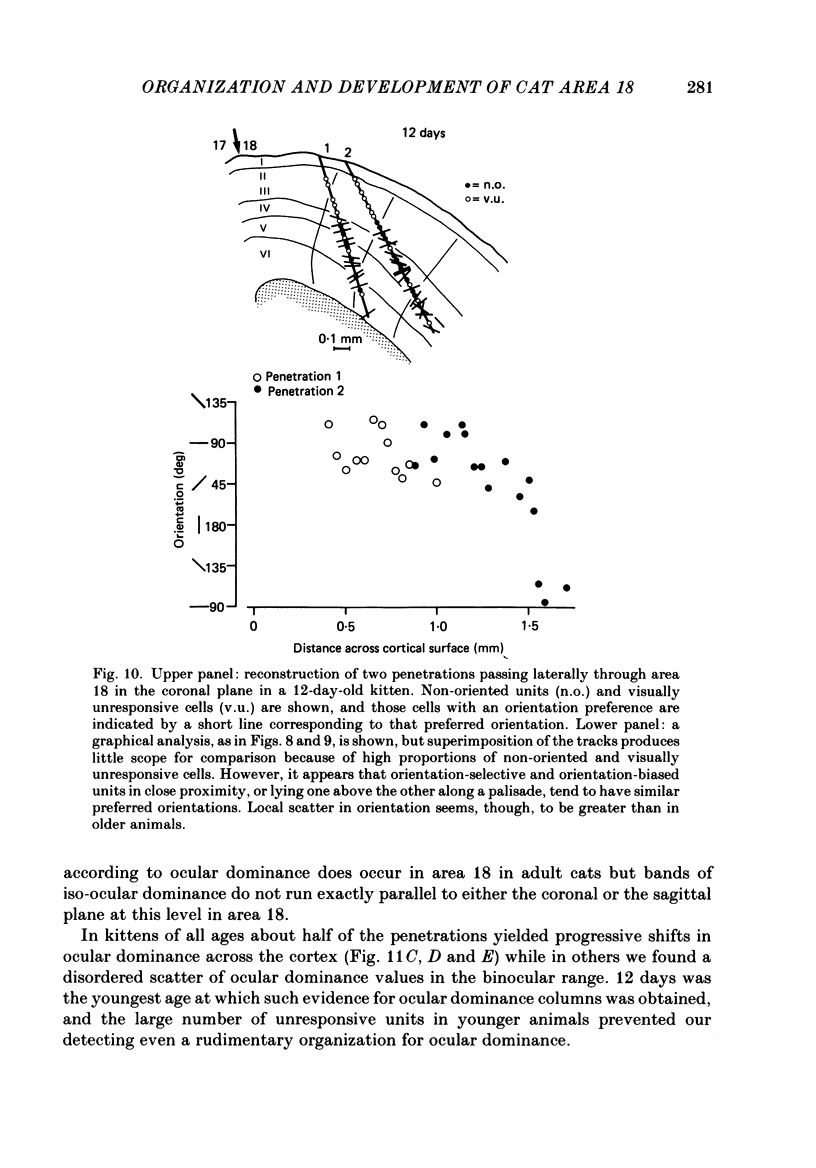
PDF






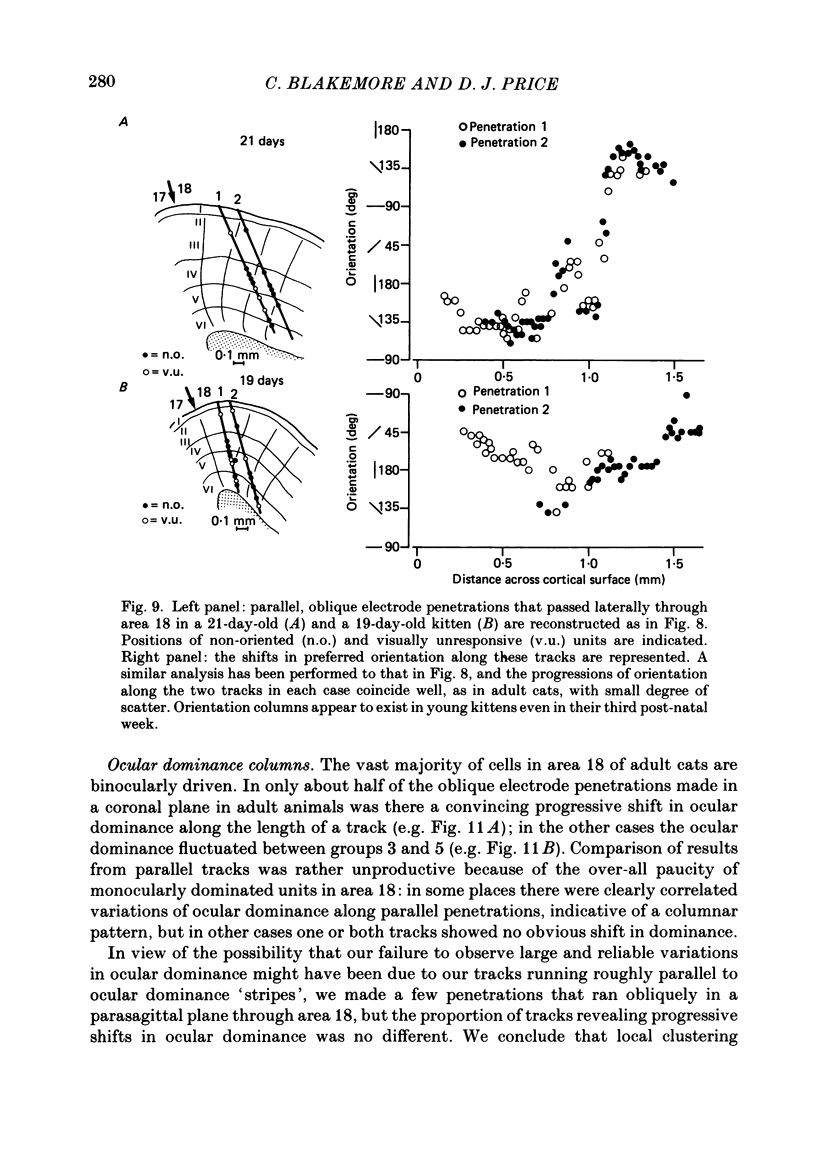







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res. 1975 Dec 22;24(2):159–179. doi: 10.1007/BF00234061. [DOI] [PubMed] [Google Scholar]
- Albus K., Beckmann R. Second and third visual areas of the cat: interindividual variability in retinotopic arrangement and cortical location. J Physiol. 1980 Feb;299:247–276. doi: 10.1113/jphysiol.1980.sp013123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus K., Sieber B. On the spatial arrangement of iso-orientation bands in the cat's visual cortical areas 17 and 18: a 14C-deoxyglucose study. Exp Brain Res. 1984;56(2):384–388. doi: 10.1007/BF00236295. [DOI] [PubMed] [Google Scholar]
- Albus K., Wolf W. Early post-natal development of neuronal function in the kitten's visual cortex: a laminar analysis. J Physiol. 1984 Mar;348:153–185. doi: 10.1113/jphysiol.1984.sp015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker R. L., Cragg B. G. Development of the extrinsic connections of the visual cortex in the cat. J Comp Neurol. 1974 Mar 1;154(1):29–41. doi: 10.1002/cne.901540103. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R. A high probability of an orientation shift between layers 4 and 5 in central parts of the cat striate cortex. Exp Brain Res. 1982;48(2):245–255. doi: 10.1007/BF00237220. [DOI] [PubMed] [Google Scholar]
- Bilge M., Bingle A., Seneviratne K. N., Whitteridge D. A map of the visual cortex in the cat. J Physiol. 1967 Jul;191(2):116P–118P. [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Mitchell D. E., Muir D. W., Pettigrew J. D. A physiological and behavioural study in cats of the effect of early visual experience with contours of a single orientation. J Physiol. 1977 Mar;265(3):615–636. doi: 10.1113/jphysiol.1977.sp011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds A. B., Freeman R. D. Development of optical quality in the kitten eye. Vision Res. 1978;18(4):391–398. doi: 10.1016/0042-6989(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Braastad B. O., Heggelund P. Development of spatial receptive-field organization and orientation selectivity in kitten striate cortex. J Neurophysiol. 1985 May;53(5):1158–1178. doi: 10.1152/jn.1985.53.5.1158. [DOI] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D. Generality of the functional structure of the neocortex. Naturwissenschaften. 1977 Oct;64(10):507–517. doi: 10.1007/BF00483547. [DOI] [PubMed] [Google Scholar]
- Daniels J. D., Pettigrew J. D., Norman J. L. Development of single-neuron responses in kitten's lateral geniculate nucleus. J Neurophysiol. 1978 Nov;41(6):1373–1393. doi: 10.1152/jn.1978.41.6.1373. [DOI] [PubMed] [Google Scholar]
- Donaldson I. M., Nash J. R. The effect of a chronic lesion in cortical area 17 on the visual responses of units in area 18 of the cat. J Physiol. 1975 Feb;245(2):325–332. doi: 10.1113/jphysiol.1975.sp010848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Cottee L. J. Visual receptive-field properties of cells in area 18 of cat's cerebral cortex before and after acute lesions in area 17. J Neurophysiol. 1975 Jul;38(4):735–750. doi: 10.1152/jn.1975.38.4.735. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J. Structure of physiologically classified neurones in the kitten dorsal lateral geniculate nucleus. Nature. 1982 Nov 11;300(5888):180–183. doi: 10.1038/300180a0. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J Physiol. 1978 May;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., Andrews D. P. Orientation tuning of cells in areas 17 and 18 of the cat's visual cortex. Exp Brain Res. 1978 Mar 15;31(3):341–351. doi: 10.1007/BF00237294. [DOI] [PubMed] [Google Scholar]
- Henderson Z. An anatomical investigation of projections from lateral geniculate nucleus to visual cortical areas 17 and 18 in newborn kitten. Exp Brain Res. 1982;46(2):177–185. doi: 10.1007/BF00237174. [DOI] [PubMed] [Google Scholar]
- Henderson Z., Blakemore C. Organization of the visual pathways in the newborn kitten. Neurosci Res. 1986 Sep;3(6):628–659. doi: 10.1016/0168-0102(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Sur M., Uhlrich D. J., Sherman S. M. Termination patterns of individual X- and Y-cell axons in the visual cortex of the cat: projections to area 18, to the 17/18 border region, and to both areas 17 and 18. J Comp Neurol. 1985 Mar 8;233(2):190–212. doi: 10.1002/cne.902330204. [DOI] [PubMed] [Google Scholar]
- Kato H., Bishop P. O., Orban G. A. Hypercomplex and simple/complex cell classifications in cat striate cortex. J Neurophysiol. 1978 Sep;41(5):1071–1095. doi: 10.1152/jn.1978.41.5.1071. [DOI] [PubMed] [Google Scholar]
- Kato N., Kawaguchi S., Yamamoto T., Samejima A., Miyata H. Postnatal development of the geniculocortical projection in the cat: electrophysiological and morphological studies. Exp Brain Res. 1983;51(1):65–72. doi: 10.1007/BF00236803. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Cortical effect of early selective exposure to diagonal lines. Science. 1975 Nov 28;190(4217):902–904. doi: 10.1126/science.1188371. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Effects of early experience upon orientation sensitivity and binocularity of neurons in visual cortex of cats. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1272–1276. doi: 10.1073/pnas.74.3.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Receptive-field properties of different classes of neurons in visual cortex of normal and dark-reared cats. J Neurophysiol. 1980 Apr;43(4):1111–1132. doi: 10.1152/jn.1980.43.4.1111. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Rabovsky J. L. Activation by sanguinarine of active sodium efflux from frog skeletal muscle in the presence of ouabain. J Physiol. 1979 Oct;295:1–20. doi: 10.1113/jphysiol.1979.sp012951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Rescaling of the retinal map of visual space during growth of the kitten's eye. Brain Res. 1980 Mar 17;186(1):55–65. doi: 10.1016/0006-8993(80)90255-3. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Callens M., Colle J. M. Unit responses to moving stimuli in area 18 of the cat. Brain Res. 1975 Jun 13;90(2):205–219. doi: 10.1016/0006-8993(75)90302-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. J., Blakemore C. Regressive events in the postnatal development of association projections in the visual cortex. Nature. 1985 Aug 22;316(6030):721–724. doi: 10.1038/316721a0. [DOI] [PubMed] [Google Scholar]
- Price D. J., Blakemore C. The postnatal development of the association projection from visual cortical area 17 to area 18 in the cat. J Neurosci. 1985 Sep;5(9):2443–2452. doi: 10.1523/JNEUROSCI.05-09-02443.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. J. Patterns of cytochrome oxidase activity in areas 17, 18 and 19 of the visual cortex of cats and kittens. Exp Brain Res. 1985;58(1):125–133. doi: 10.1007/BF00238960. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Lindström S., Wiesel T. N. The distribution of afferents representing the right and left eyes in the cat's visual cortex. Brain Res. 1977 Aug 5;131(1):103–116. doi: 10.1016/0006-8993(77)90031-2. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Cat parastriate cortex: a primary or secondary visual area. J Neurophysiol. 1975 Sep;38(5):1099–1113. doi: 10.1152/jn.1975.38.5.1099. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Rosenquist A. C., Palmer L. A. Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol. 1979 Jun 15;185(4):657–678. doi: 10.1002/cne.901850405. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]


