Abstract
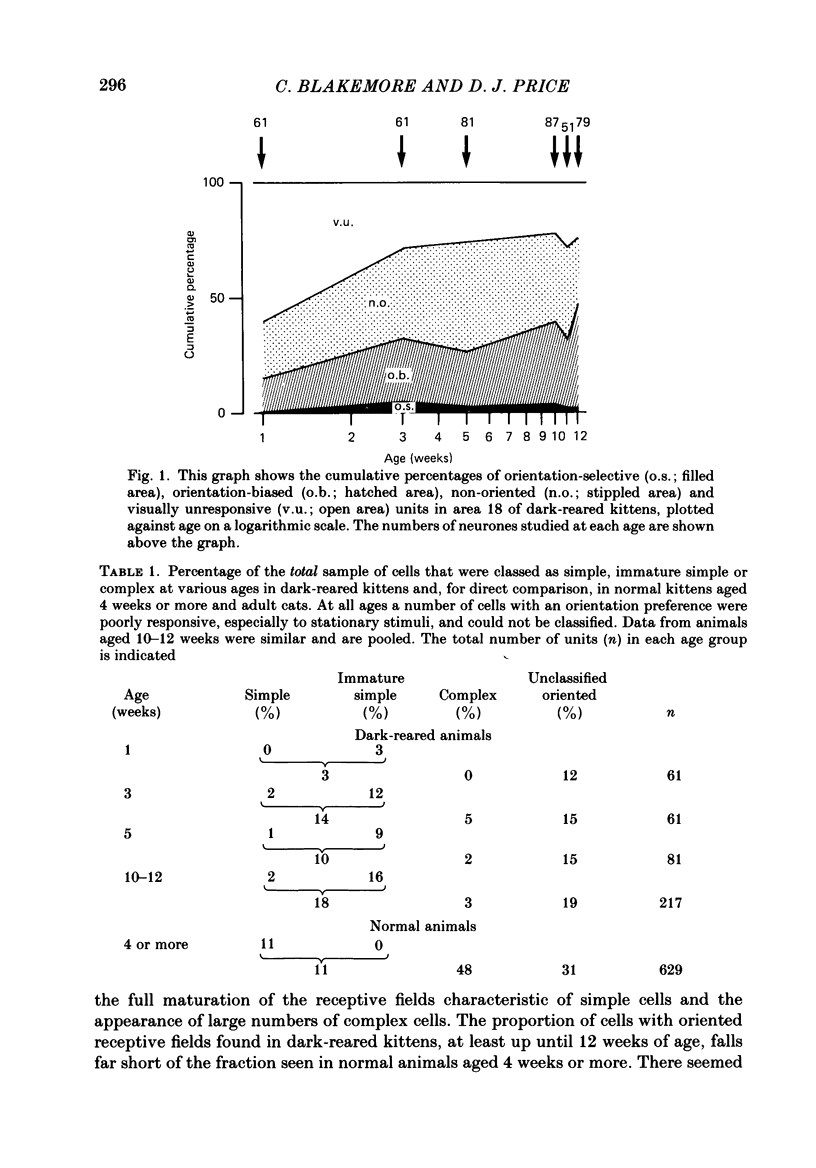
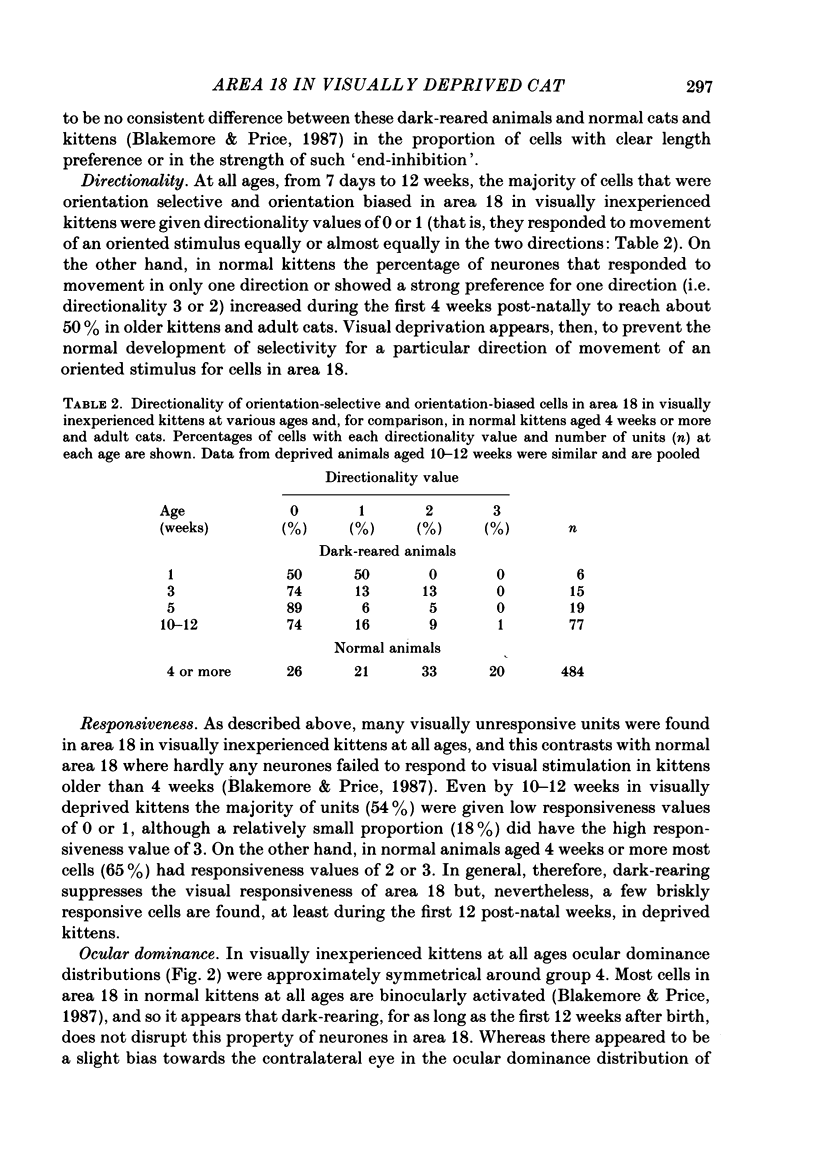
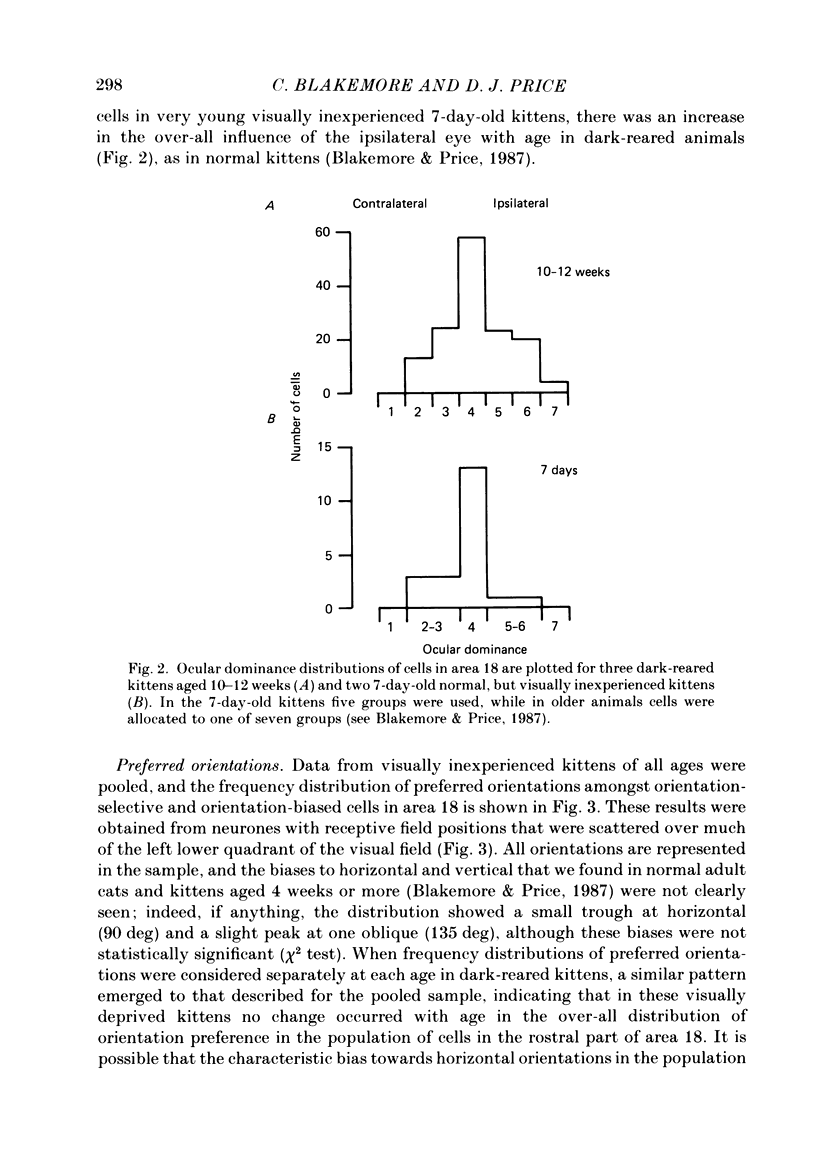
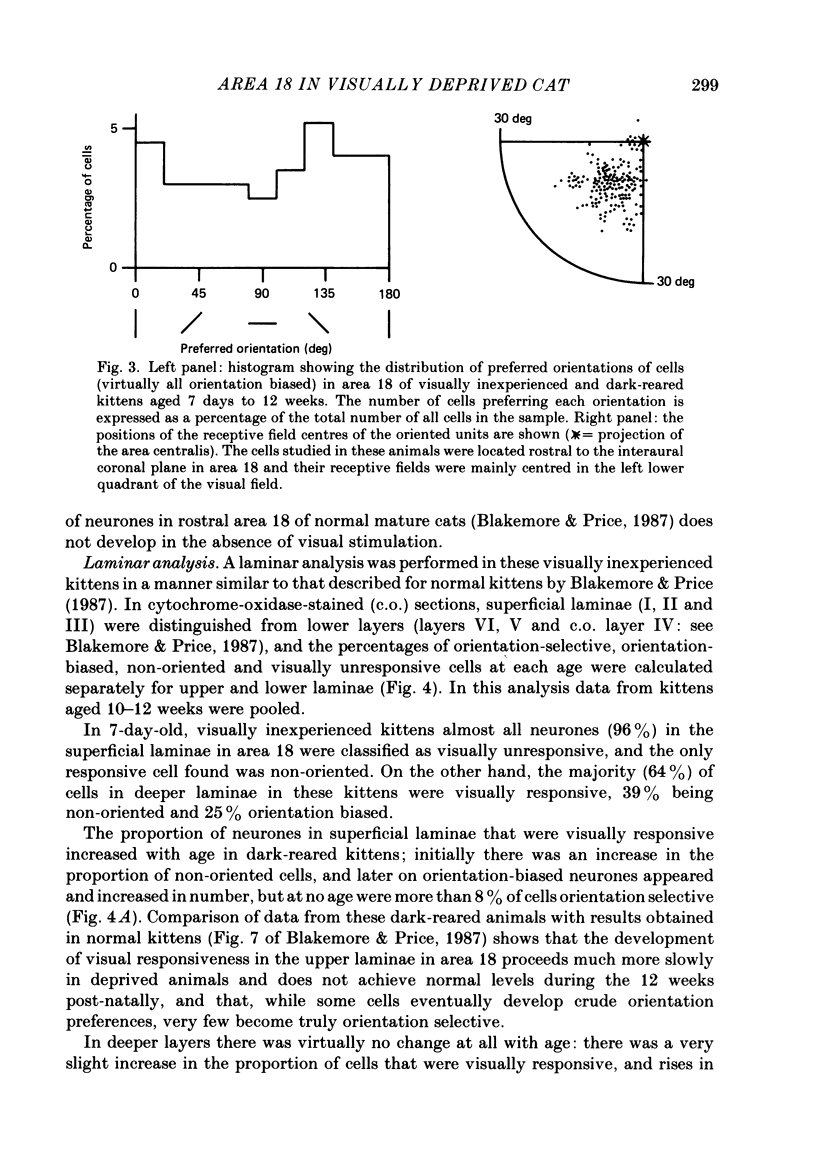
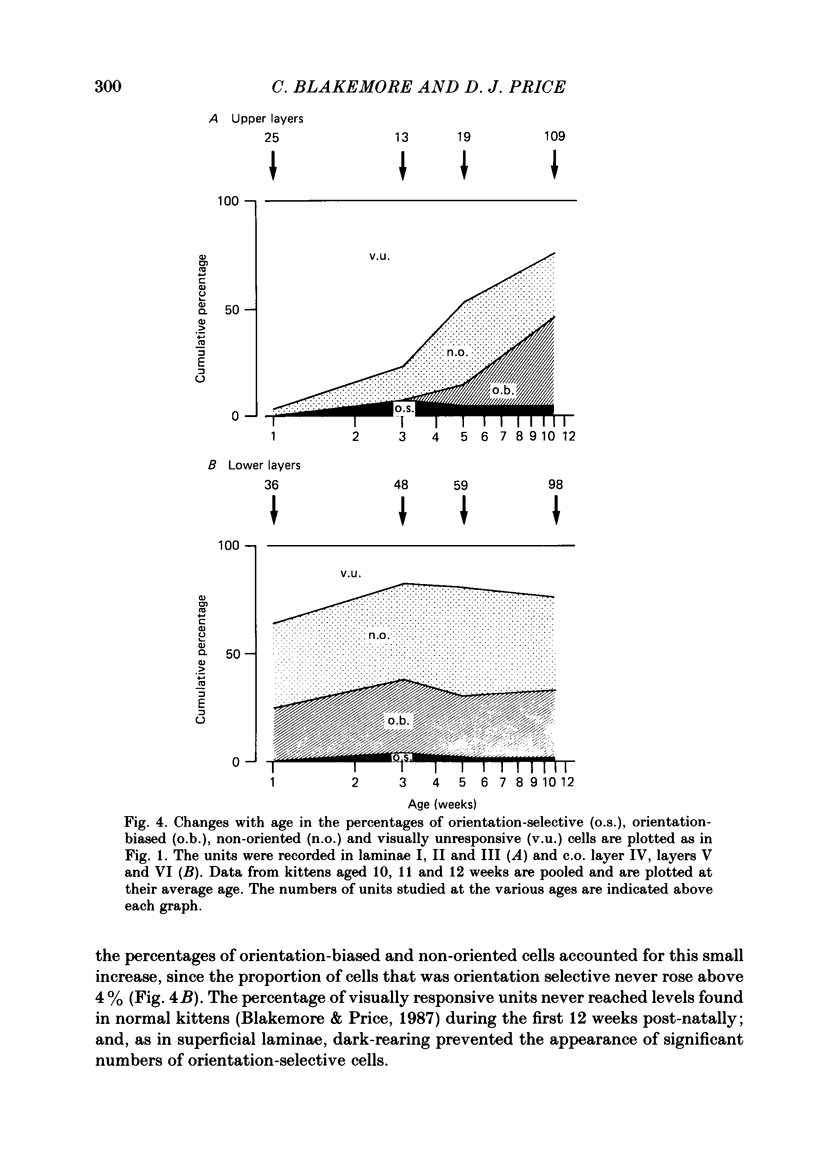
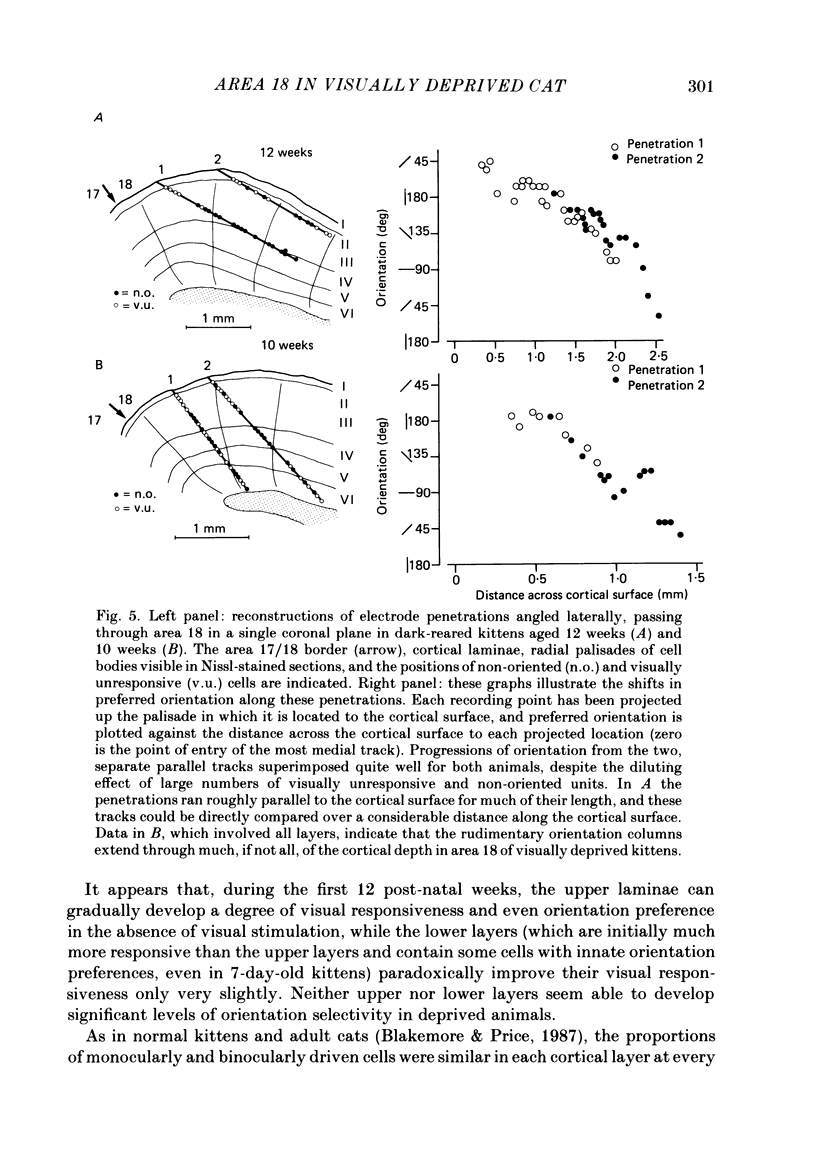
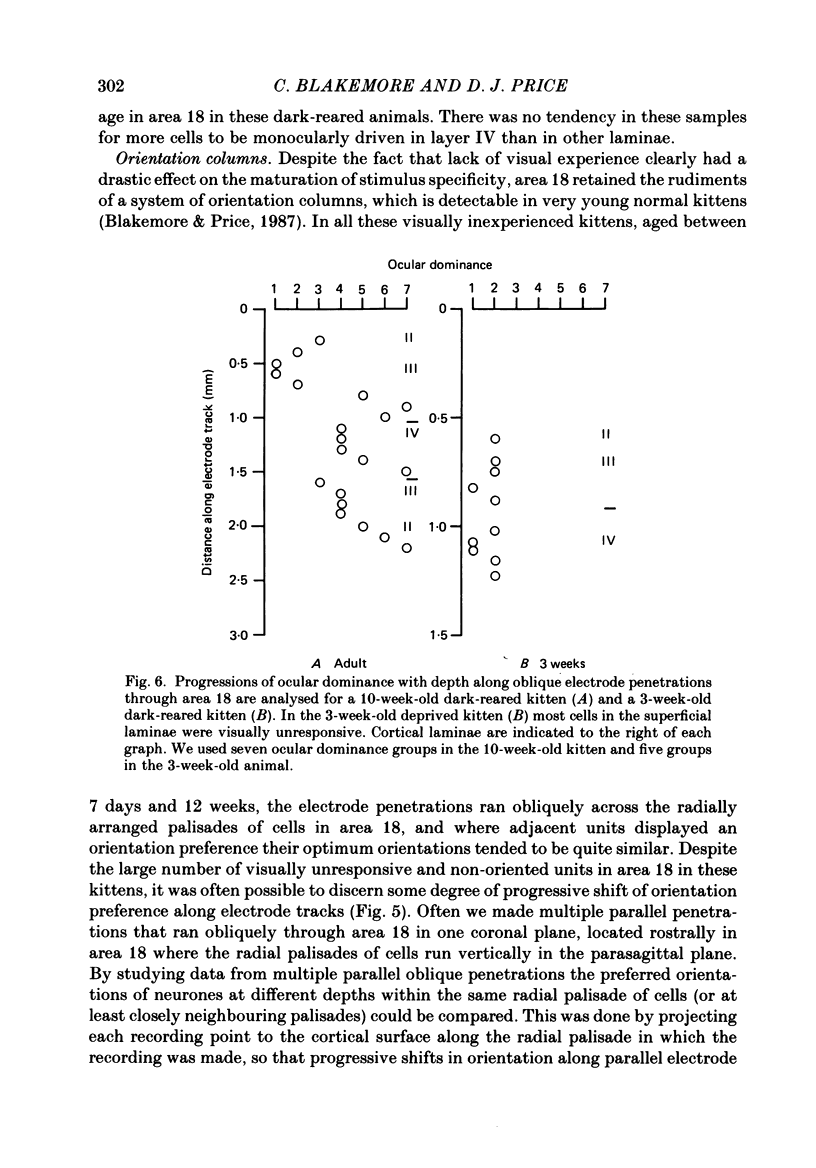
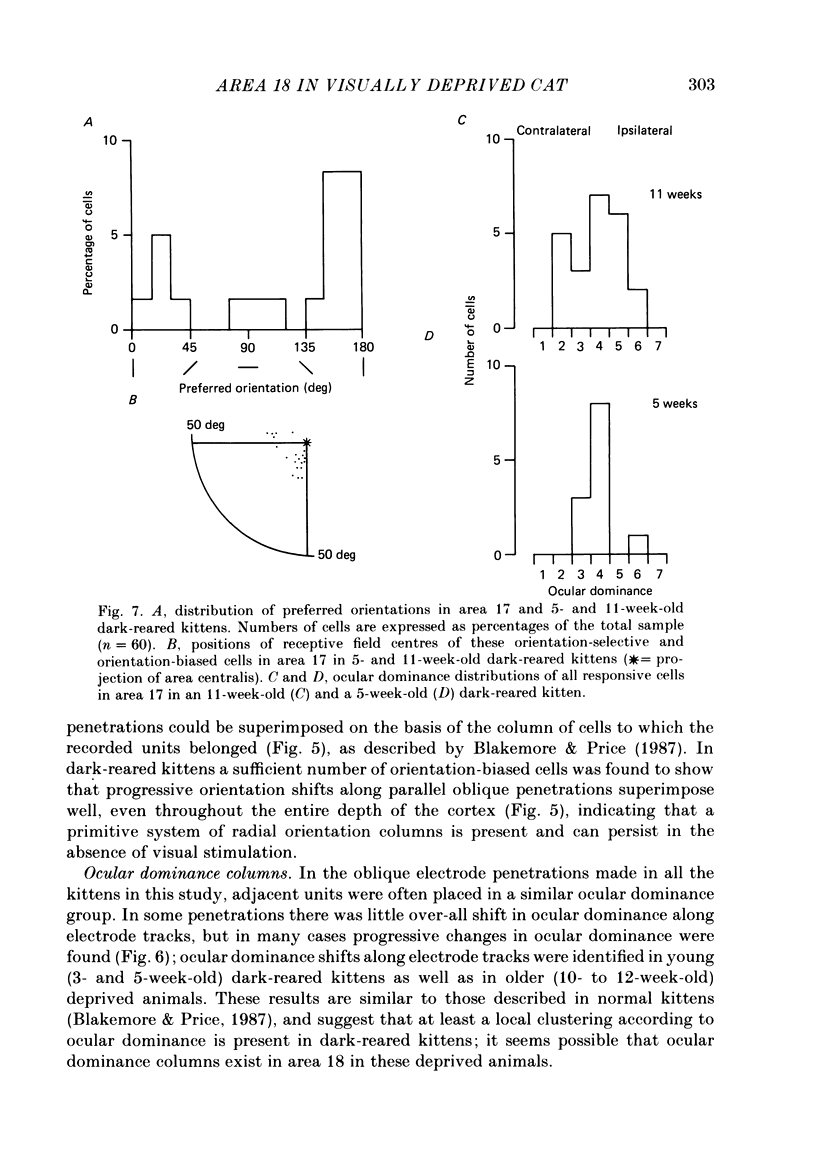
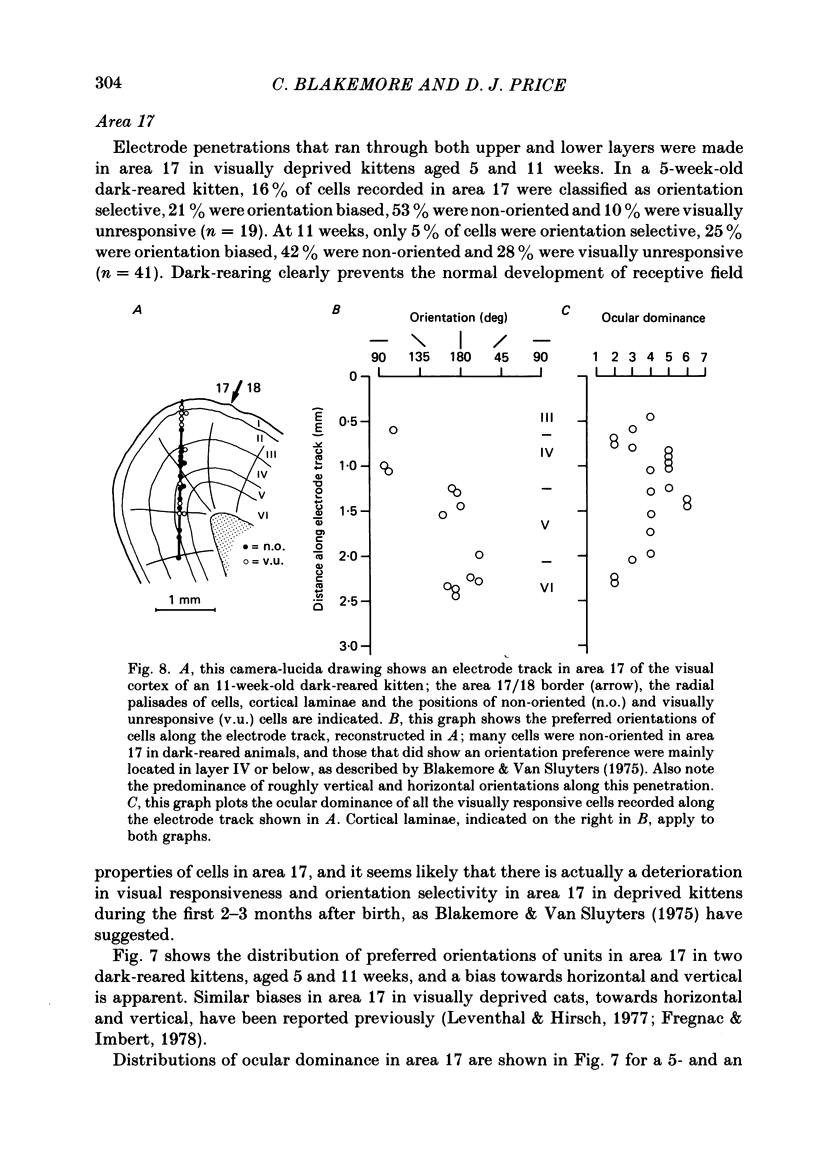
1. We recorded extracellularly from 420 single units in area 18 in visually inexperienced kittens aged 7 days and dark-reared kittens aged between 3 and 12 weeks and from 60 single units in area 17 in dark-reared kittens aged 5 and 11 weeks. 2. Visual deprivation generally depressed the maturation of area 18, although some features were affected more than others and certain developmental improvements still occurred. The percentage of visually responsive units in area 18 increased from 40% in 7-day-old kittens to about 75% in dark-reared animals 10-12 weeks. At each age a proportion of cells was orientation biased (between 15 and 45%) and these neurones appeared to be arranged in a crude columnar fashion. However, dark-rearing, from birth, prevented the development of a significant proportion of orientation-selective cells in area 18; no more than 5% of neurones were orientation-selective at any age. We found no major bias in the over-all distribution of preferred orientations of cells in area 18 in dark-reared kittens. 3. Simple cells, which are found in area 18 even in very young, visually inexperienced kittens, persisted after dark-rearing, although most retained immature properties. Relatively few complex cells were found in area 18 in visually deprived animals. 4. The majority of neurones in area 18 of dark-reared kittens were binocularly driven, many equally well by either eye; evidence for regional variation in ocular dominance (indicative of a columnar pattern) was found in these deprived animals. 5. A laminar analysis in area 18 showed that percentages of non-oriented and orientation-biased cells changed little, if at all, in lower laminae (IV, V, and VI) but increased substantially in upper layers (above layer IV) in the absence of visual stimulation, over the first 12 post-natal weeks. 6. A comparison of the effects of dark-rearing on areas 17 and 18 indicates that the normal development of visual responsiveness and specific receptive field properties is suppressed in both areas during the first 12 post-natal weeks. It is possible that area 17 has a greater degree of orientation selectivity than area 18 in young visually deprived kittens and this may reflect a difference in the type of afferent inputs.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K., Wolf W. Early post-natal development of neuronal function in the kitten's visual cortex: a laminar analysis. J Physiol. 1984 Mar;348:153–185. doi: 10.1113/jphysiol.1984.sp015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker R. L., Cragg B. G. Development of the extrinsic connections of the visual cortex in the cat. J Comp Neurol. 1974 Mar 1;154(1):29–41. doi: 10.1002/cne.901540103. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Price D. J. The organization and post-natal development of area 18 of the cat's visual cortex. J Physiol. 1987 Mar;384:263–292. doi: 10.1113/jphysiol.1987.sp016454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976 May 28;25(2):139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J Physiol. 1978 May;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Henderson Z., Blakemore C. Organization of the visual pathways in the newborn kitten. Neurosci Res. 1986 Sep;3(6):628–659. doi: 10.1016/0168-0102(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Imbert M., Buisseret P. Receptive field characteristics and plastic properties of visual cortical cells in kittens reared with or without visual experience. Exp Brain Res. 1975;22(1):25–36. doi: 10.1007/BF00235409. [DOI] [PubMed] [Google Scholar]
- Kato N., Kawaguchi S., Yamamoto T., Samejima A., Miyata H. Postnatal development of the geniculocortical projection in the cat: electrophysiological and morphological studies. Exp Brain Res. 1983;51(1):65–72. doi: 10.1007/BF00236803. [DOI] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Effects of early experience upon orientation sensitivity and binocularity of neurons in visual cortex of cats. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1272–1276. doi: 10.1073/pnas.74.3.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Receptive-field properties of different classes of neurons in visual cortex of normal and dark-reared cats. J Neurophysiol. 1980 Apr;43(4):1111–1132. doi: 10.1152/jn.1980.43.4.1111. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. J. Patterns of cytochrome oxidase activity in areas 17, 18 and 19 of the visual cortex of cats and kittens. Exp Brain Res. 1985;58(1):125–133. doi: 10.1007/BF00238960. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F. Receptive-field properties and neuronal connectivity in striate and parastriate cortex of contour-deprived cats. J Neurophysiol. 1976 May;39(3):613–630. doi: 10.1152/jn.1976.39.3.613. [DOI] [PubMed] [Google Scholar]
- Spear P. D., Tong L., Langsetmo A. Striate cortex neurons of binocularly deprived kittens respond to visual stimuli through the closed eyelids. Brain Res. 1978 Oct 20;155(1):141–146. doi: 10.1016/0006-8993(78)90315-3. [DOI] [PubMed] [Google Scholar]
- Swindale N. V. Absence of ocular dominance patches in dark-reared cats. Nature. 1981 Mar 26;290(5804):332–333. doi: 10.1038/290332a0. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Rosenquist A. C., Palmer L. A. Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol. 1979 Jun 15;185(4):657–678. doi: 10.1002/cne.901850405. [DOI] [PubMed] [Google Scholar]


