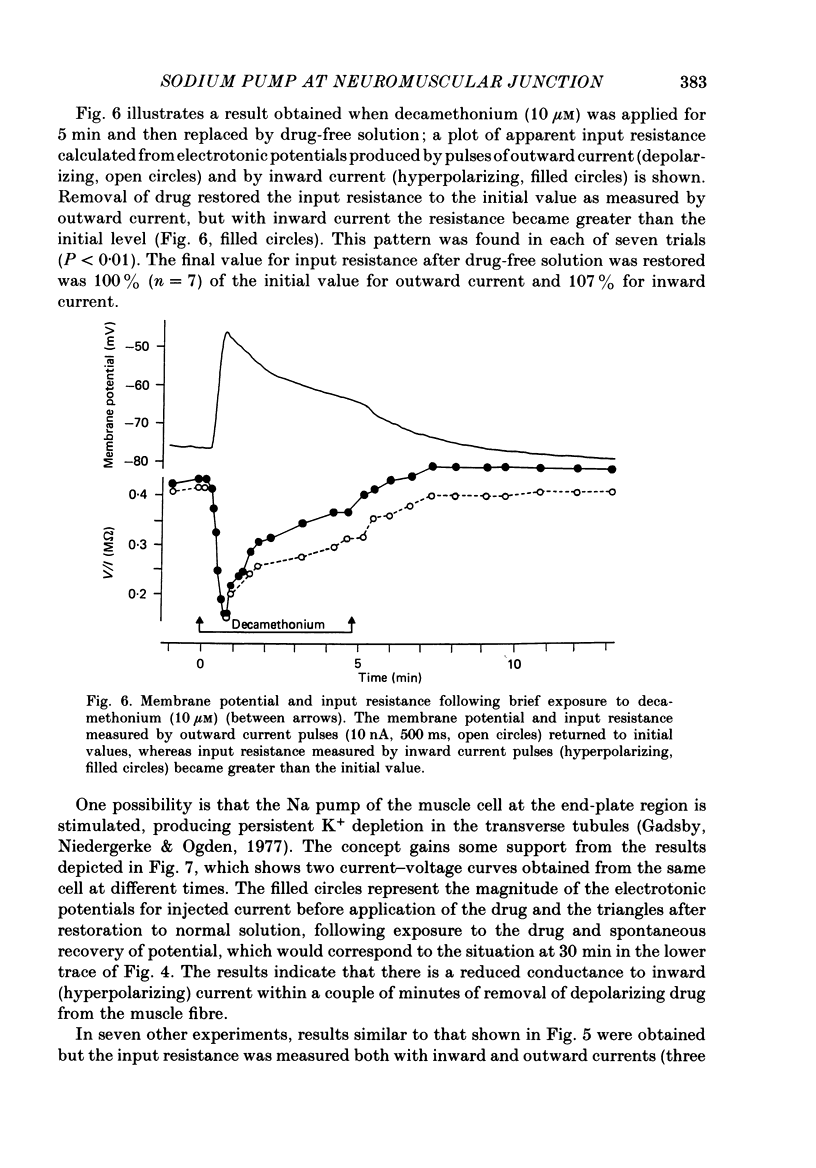
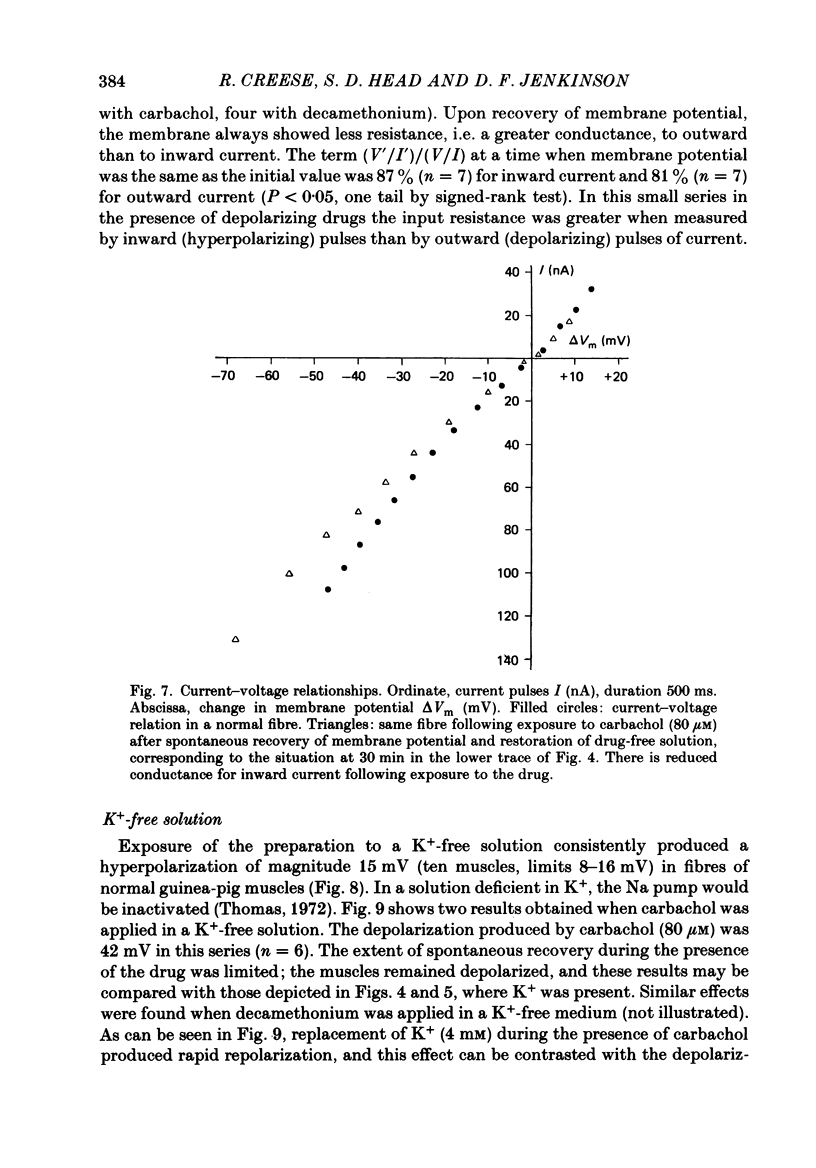
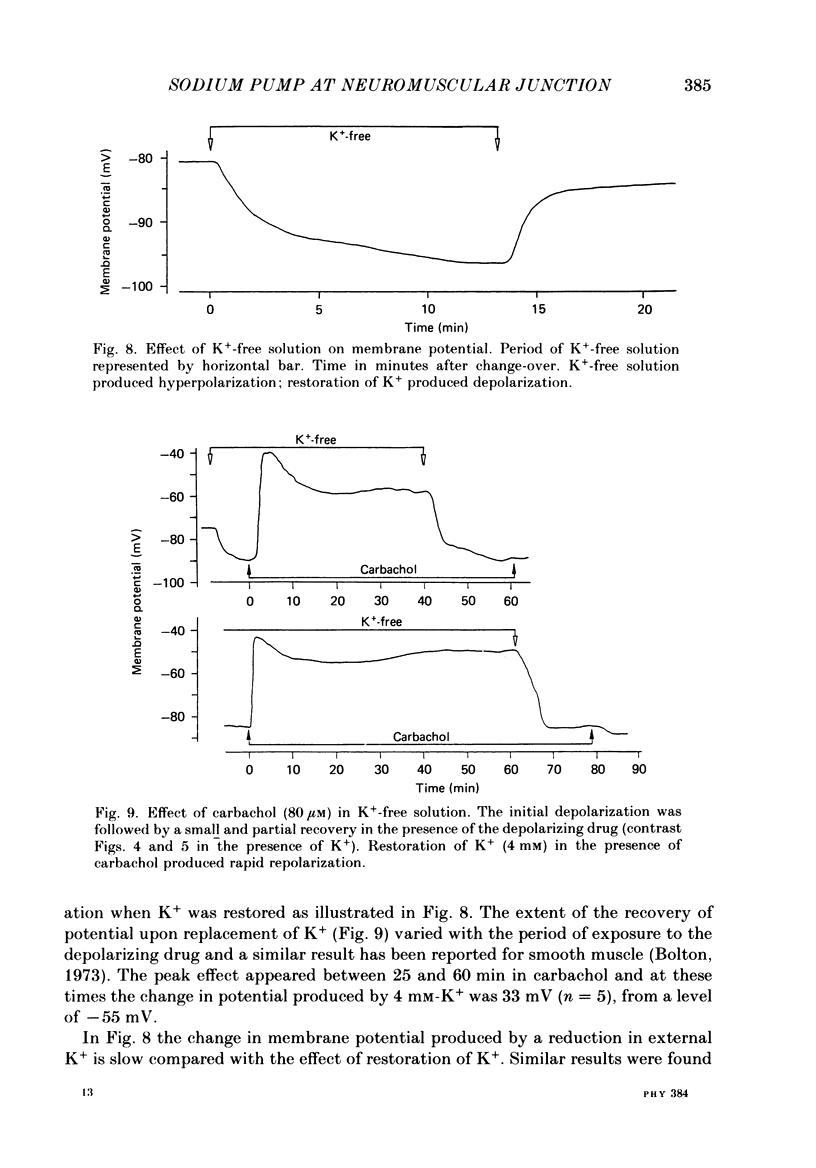
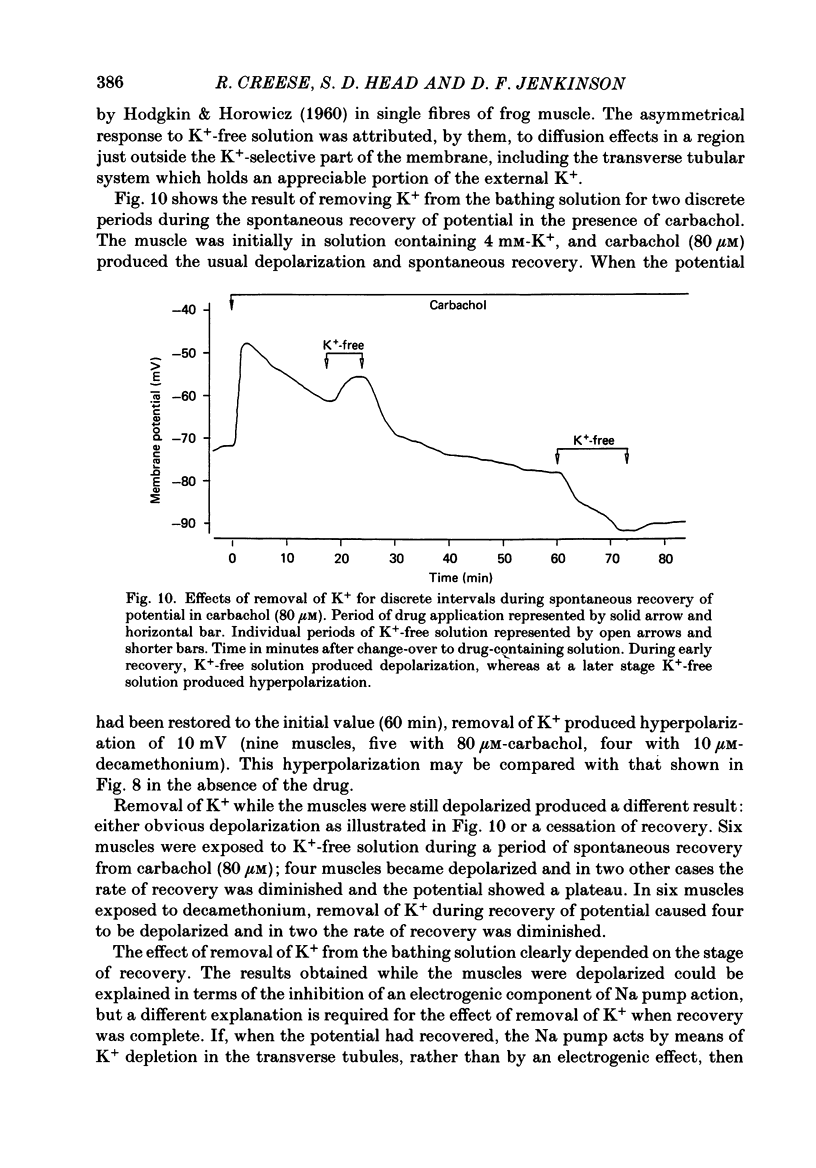
Abstract
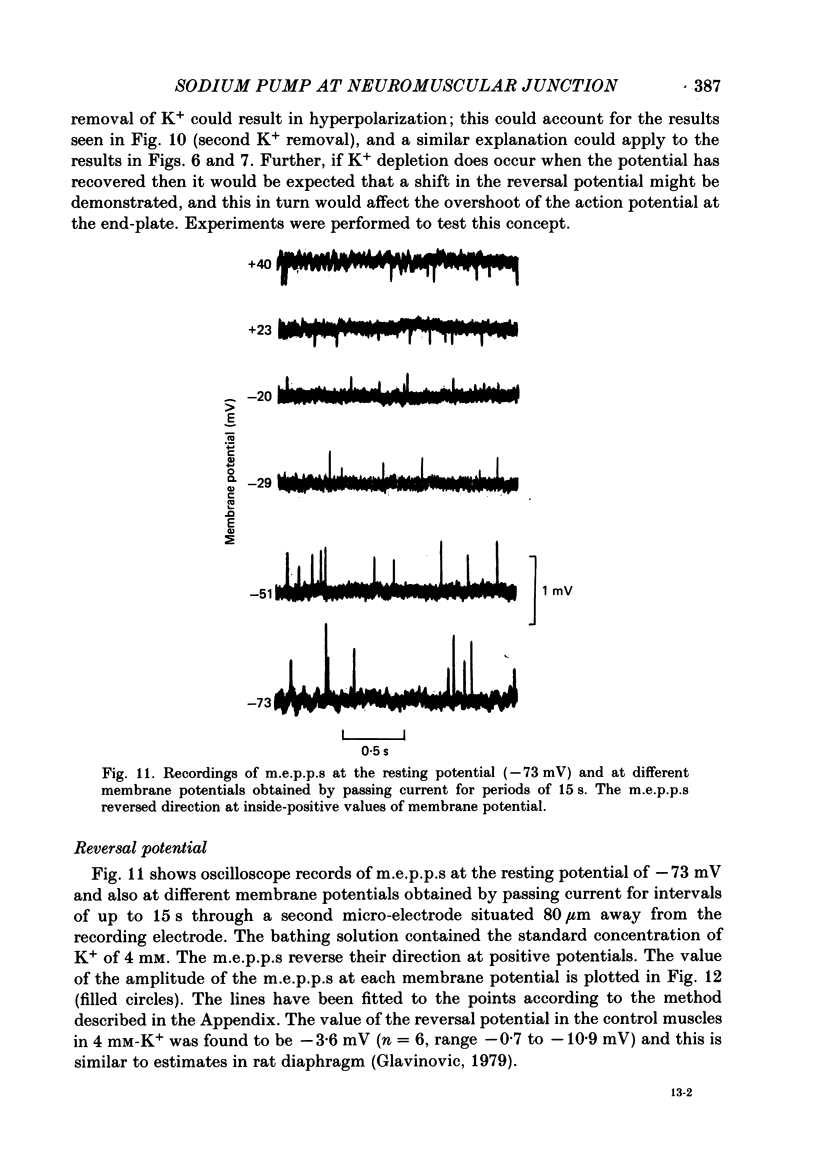
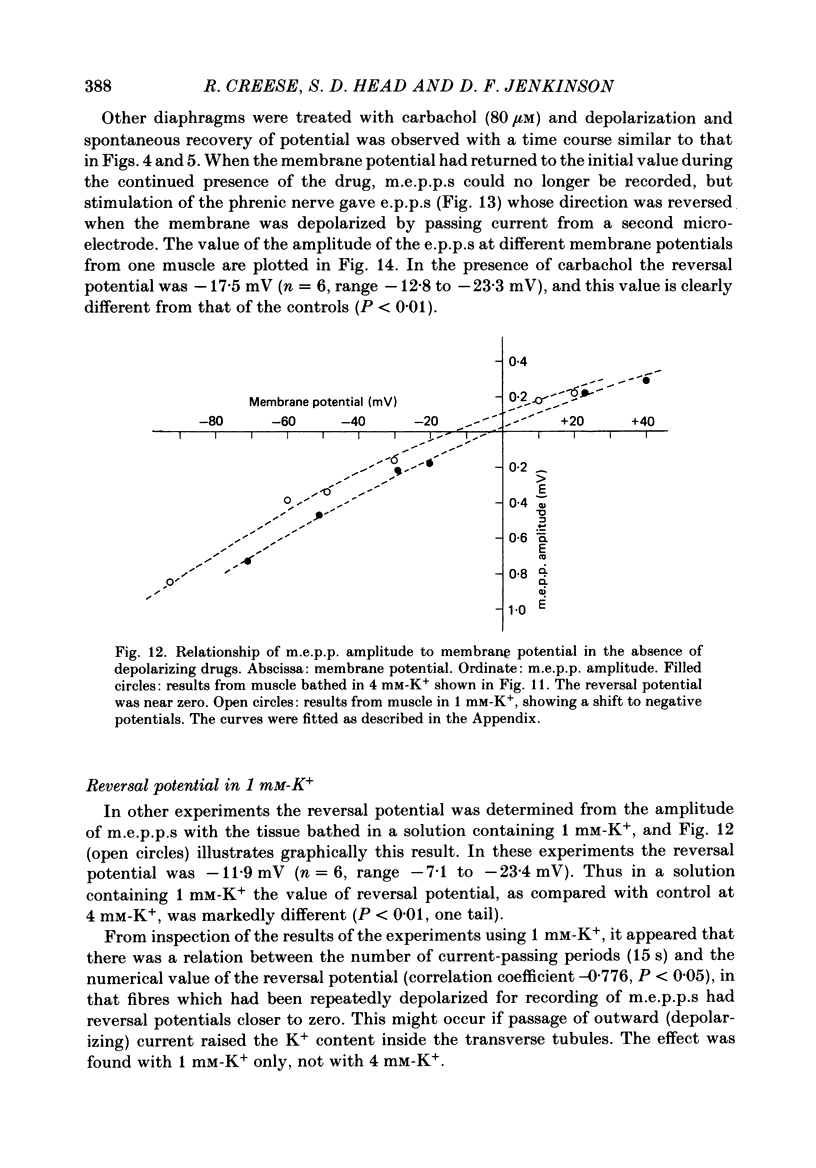
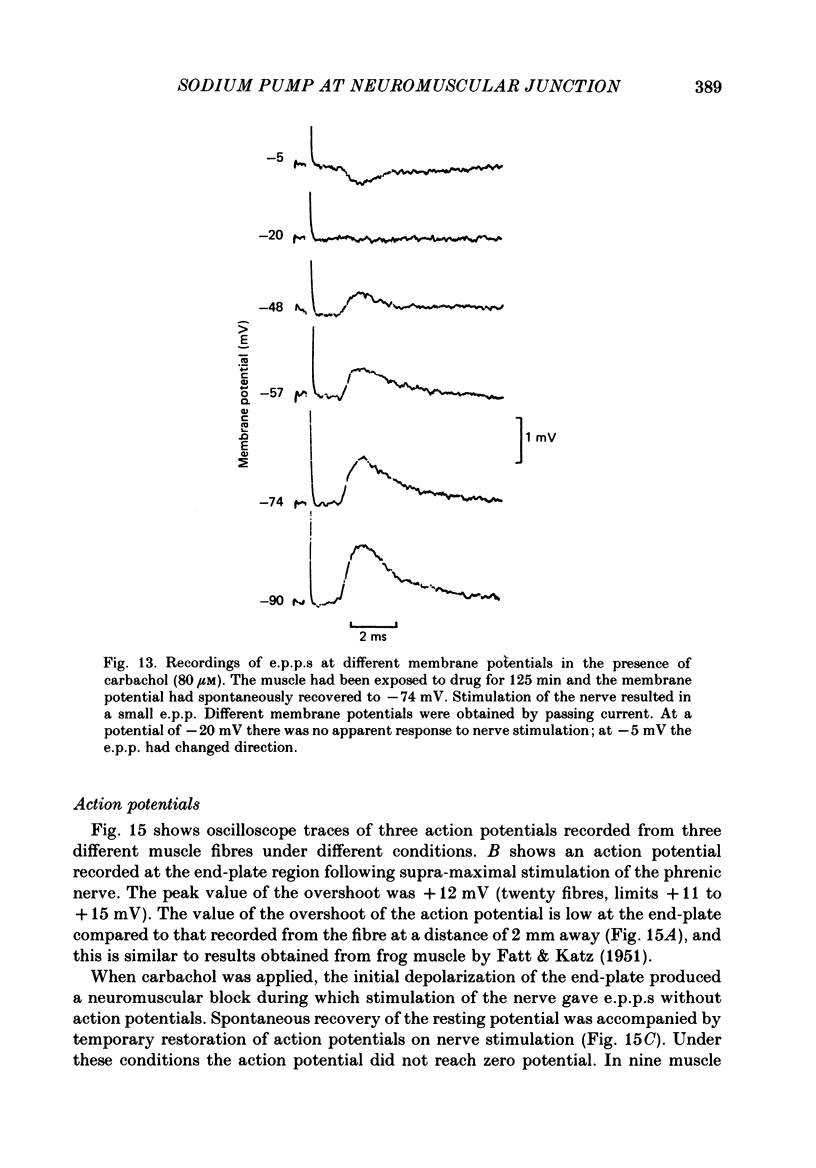
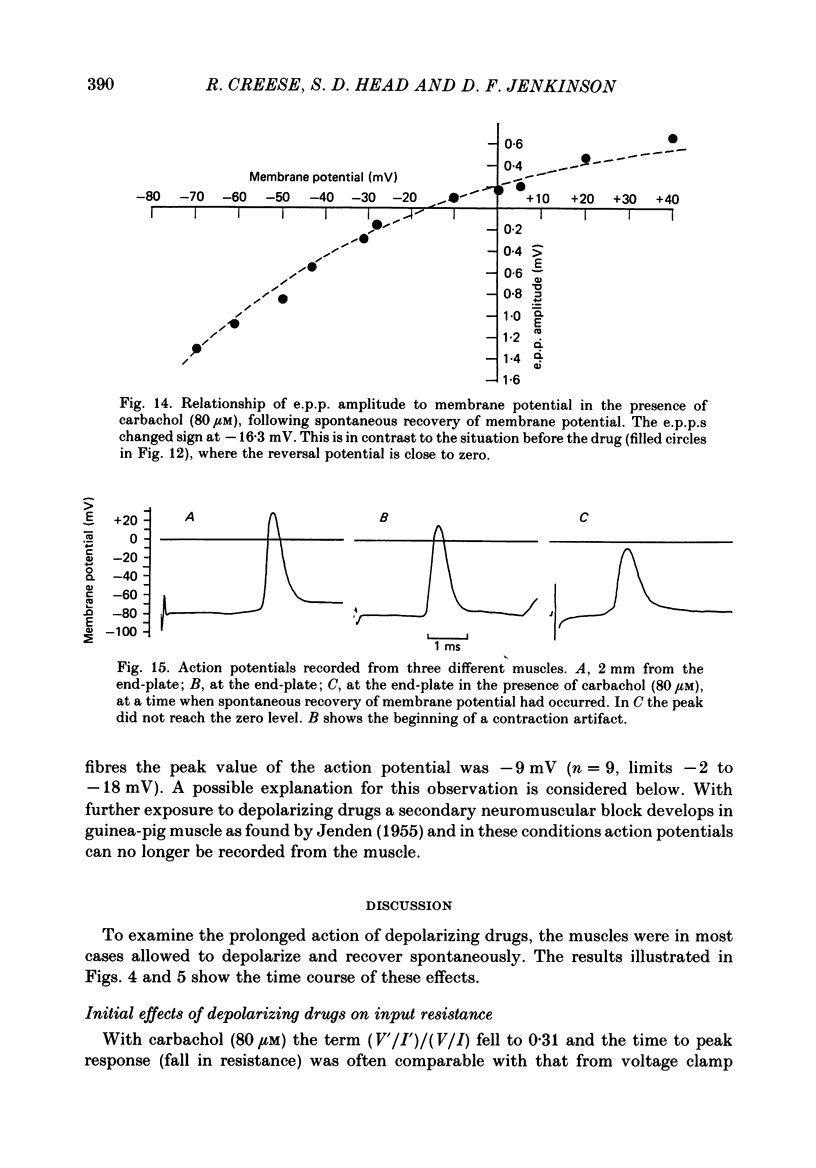
1. Depolarization caused by carbachol or decamethonium is followed by spontaneous recovery of membrane potential in the presence of the drug. The involvement of the Na pump in this recovery has been investigated in guinea-pig diaphragm at 37 degrees C. 2. Restoration of potassium ions (K+) to the bathing solution gives a rapid recovery of membrane potential which is compatible with a component of recovery of potential being attributable to an electrogenic ion pump and from which a Na pump current of over 60 nA has been estimated. 3. The maintenance of membrane potential in the presence of depolarizing drugs is interpreted in terms of a residual rate of channel opening at a time when the membrane potential is restored, balanced by Na pump action producing tubular depletion of K+. To account for these results a Na pump conductance has been added to a model circuit of drug action. 4. The peak end-plate current produced by carbachol (80 microM) is 100 nA (n = 11) as recorded by the voltage clamp technique; similar estimates may be obtained from measurements of input resistance which falls to 31% of the initial value (n = 5). In muscles desensitized by carbachol for 30 min the end-plate current is 11 nA. 5. In normal muscle removal of K+ from the bathing solution produces a reversible hyperpolarization. In muscles where the membrane potential has recovered in the continued presence of the drug, a hyperpolarization is also found on removal of K+. Withdrawal of K+ during the early stage of spontaneous recovery of potential produces a depolarization or an arrest of the spontaneous repolarization. These results are interpreted in terms of the Na pump producing different effects during the course of spontaneous repolarization. 6. Indirect evidence for K+ depletion in the transverse tubules by the Na pump is provided by an increased resistance to inward current following brief exposure to carbachol or decamethonium. A similar mechanism is used to interpret both the observed change in end-plate revérsal potential to a more negative value and the marked diminution in the amplitude of the action potential at the end-plate during drug action.
Full text
PDF

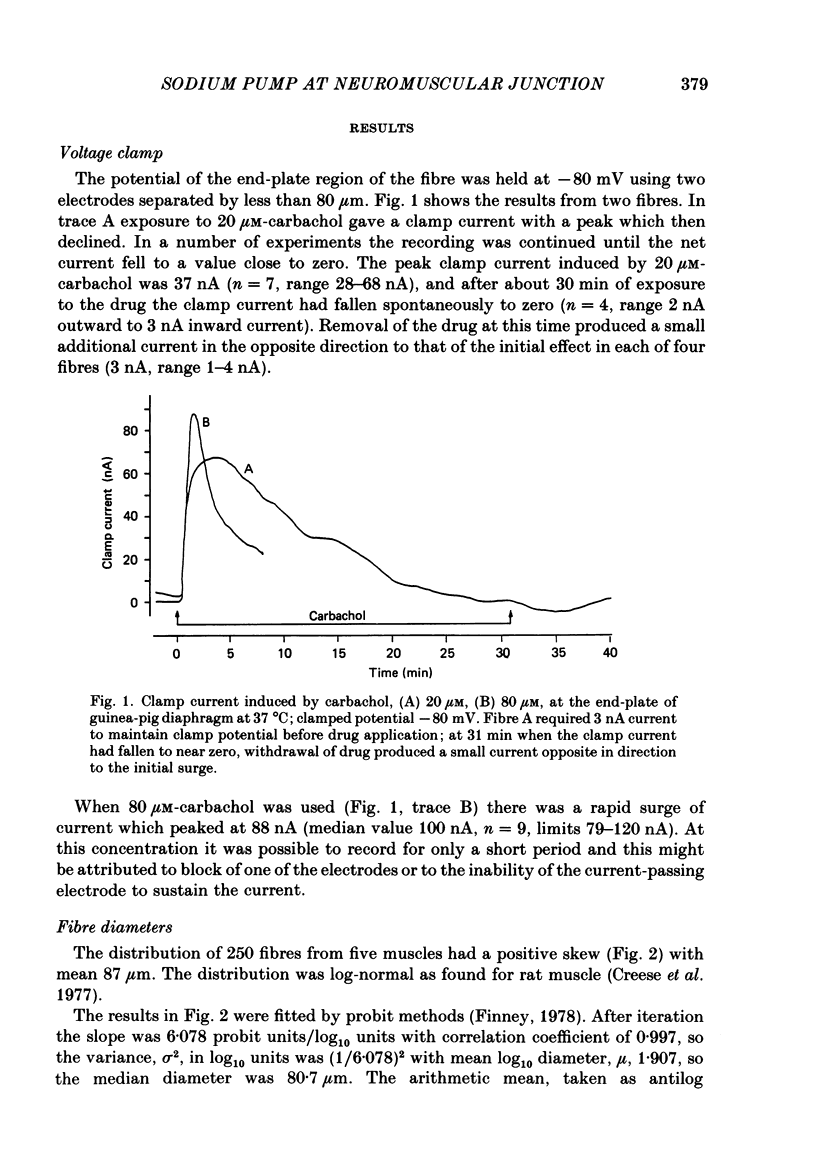
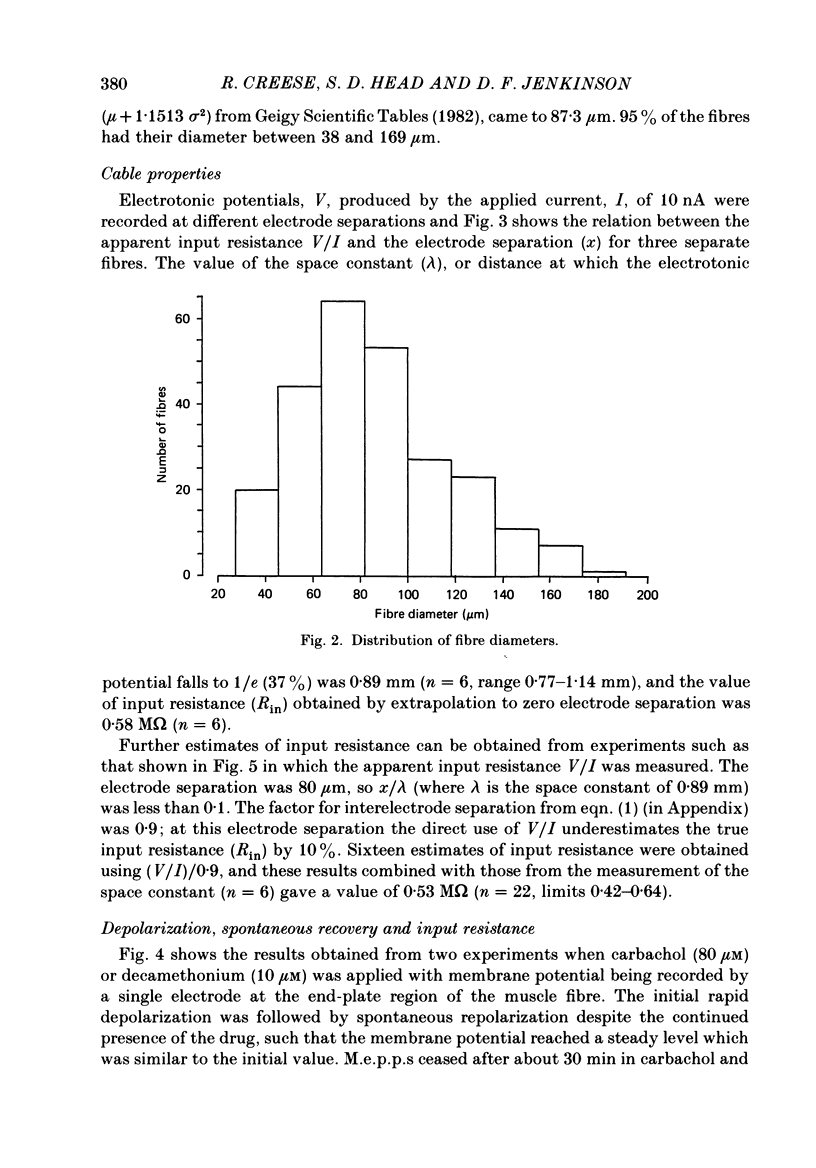
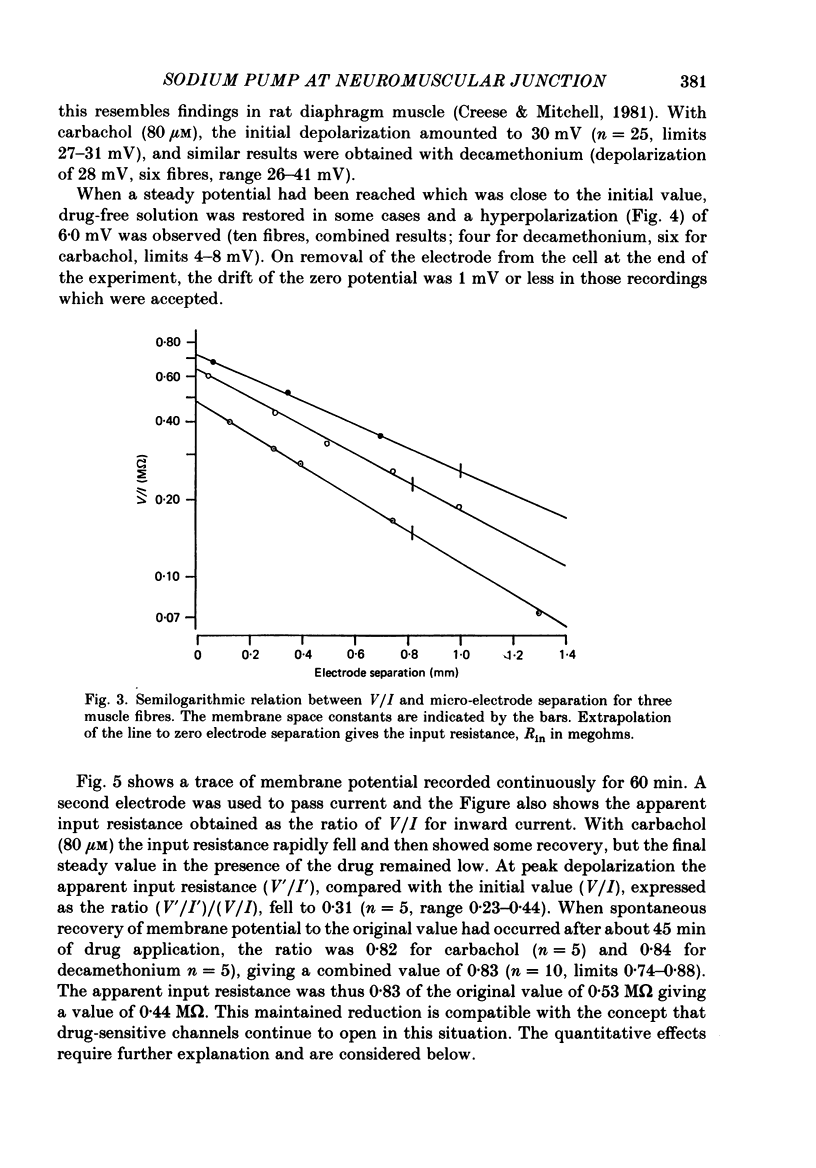
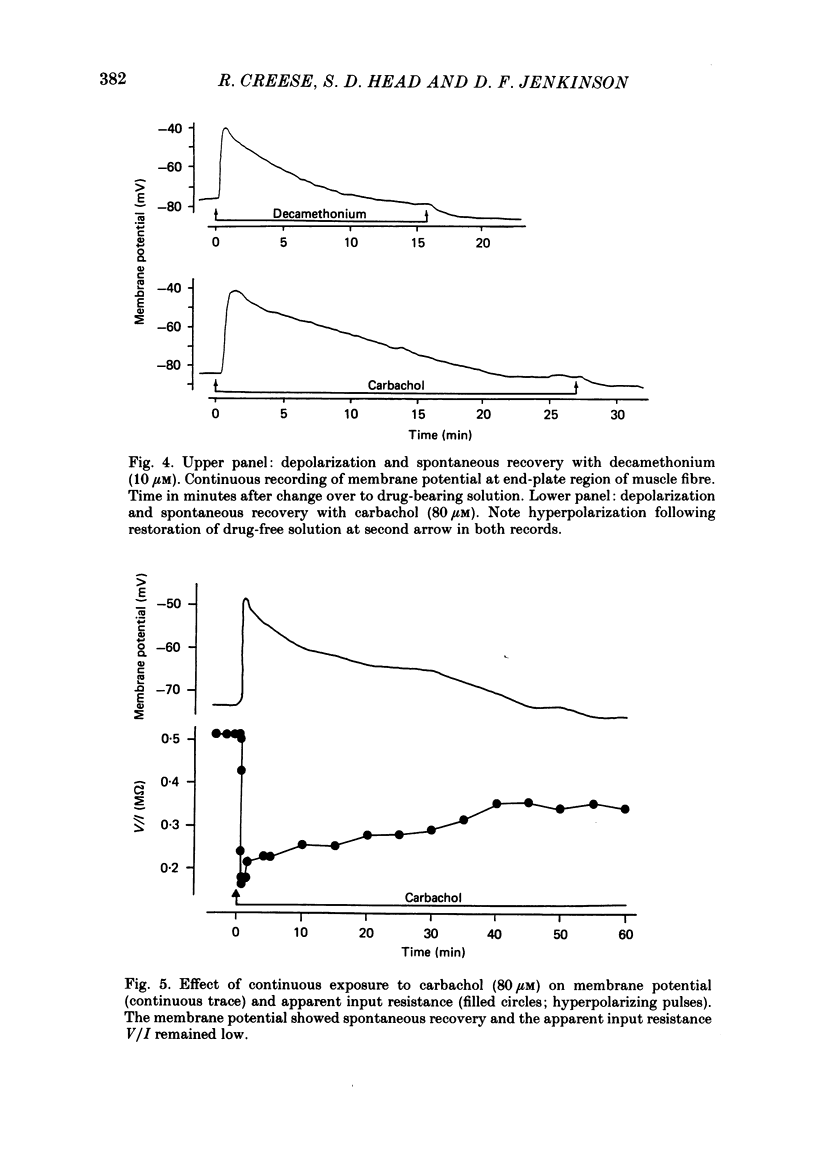












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Membrane constants of mammalian muscle fibres. J Physiol. 1959 Oct;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., PATON W. D. M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J Physiol. 1951 Sep;115(1):41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Kinnamon S. C. Increased sodium conductance in the synaptic region of rat skeletal muscle fibres. J Physiol. 1984 Jul;352:189–202. doi: 10.1113/jphysiol.1984.sp015286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Franklin G. I., Mitchell L. D. Sodium entry in rat diaphragm induced by depolarizing drugs. J Physiol. 1977 Nov;272(2):295–316. doi: 10.1113/jphysiol.1977.sp012045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Franklin G. I., Mitchell L. D. Two mechanisms for spontaneous recovery from depolarising drugs in rat muscle. Nature. 1976 Jun 3;261(5559):416–417. doi: 10.1038/261416a0. [DOI] [PubMed] [Google Scholar]
- Creese R., Humphrey P. P., Mitchell L. D. Recovery from decamethonium in rat muscle and denervated guinea-pig diaphragm. J Physiol. 1983 Jan;334:365–377. doi: 10.1113/jphysiol.1983.sp014499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Mitchell L. D. Spontaneous recovery from depolarizing drugs in rat diaphragm. J Physiol. 1981;313:173–186. doi: 10.1113/jphysiol.1981.sp013657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Rakowski R. F. Current generated by backward-running electrogenic Na pump in squid giant axons. 1984 May 31-Jun 6Nature. 309(5967):450–452. doi: 10.1038/309450a0. [DOI] [PubMed] [Google Scholar]
- Evans R. H. The entry of labelled calcium into the innervated region of the mouse diaphragm muscle. J Physiol. 1974 Aug;240(3):517–533. doi: 10.1113/jphysiol.1974.sp010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S. SODIUM PUMP: ITS ELECTRICAL EFFECTS IN SKELETAL MUSCLE. Science. 1965 Mar 19;147(3664):1442–1443. doi: 10.1126/science.147.3664.1442. [DOI] [PubMed] [Google Scholar]
- Feltz A., Jaoul A. Direct estimates of chloride activity in muscle fibres depolarized by carbachol. Br J Pharmacol. 1974 Jun;51(2):304–306. doi: 10.1111/j.1476-5381.1974.tb09661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Niedergerke R., Ogden D. C. The dual nature of the membrane potential increase associated with the activity of the sodium/potassium exchange pump in skeletal muscle fibres. Proc R Soc Lond B Biol Sci. 1977 Sep 19;198(1133):463–472. doi: 10.1098/rspb.1977.0110. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. An analysis of the relationship between the current and potential generated by a quantum of acetylcholine in muscle fibers without transverse tubules. J Membr Biol. 1973;12(3):247–272. doi: 10.1007/BF01870004. [DOI] [PubMed] [Google Scholar]
- Geduldig D. A ouabain-sensitive membrane conductance. J Physiol. 1968 Feb;194(2):521–533. doi: 10.1113/jphysiol.1968.sp008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Glavinović M. I. Voltage clamping of unparalysed cut rat diaphragm for study of transmitter release. J Physiol. 1979 May;290(2):467–480. doi: 10.1113/jphysiol.1979.sp012784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M. The electrogenic sodium pump. Soc Gen Physiol Ser. 1984;38:33–48. [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head S. D. Temperature and end-plate currents in rat diaphragm. J Physiol. 1983 Jan;334:441–459. doi: 10.1113/jphysiol.1983.sp014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENDEN D. J. The effect of drugs upon neuromuscular transmission in the isolated guinea pig diaphragm. J Pharmacol Exp Ther. 1955 Aug;114(4):398–408. [PubMed] [Google Scholar]
- Jenkinson D. H., Terrar D. A. Influence of chloride ions on changes in membrane potential during prolonged application of carbachol to frog skeletal muscle. Br J Pharmacol. 1973 Feb;47(2):363–376. doi: 10.1111/j.1476-5381.1973.tb08334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The reversal potential at the desensitized endplate. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):329–334. doi: 10.1098/rspb.1977.0144. [DOI] [PubMed] [Google Scholar]
- Kononenko N. I., Kostyuk P. G. Further studies of the potential-dependence of the sodium-induced membrane current in snail neurones. J Physiol. 1976 Apr;256(3):601–615. doi: 10.1113/jphysiol.1976.sp011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Potential-dependent membrane current during the active transport of ions in snail neurones. J Physiol. 1972 Oct;226(2):373–392. doi: 10.1113/jphysiol.1972.sp009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. H., Spannbauer P. M., Parsons R. L. Desensitisation does not selectively alter sodium channels. Nature. 1977 Aug 11;268(5620):553–555. doi: 10.1038/268553a0. [DOI] [PubMed] [Google Scholar]
- Levitt D. G. The mechanism of the sodium pump. Biochim Biophys Acta. 1980 Dec 31;604(3):321–345. doi: 10.1016/0005-2736(80)90577-5. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHOLL E. Elektrophysiologische Untersuchung der einzelnen Faseranteile des isolierten Rattenzwerchfelles. Pflugers Arch. 1957;264(5):467–483. doi: 10.1007/BF00369810. [DOI] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padjen A. L., Smith P. A. The role of the electrogenic sodium pump in the glutamate afterhyperpolarization of frog spinal cord. J Physiol. 1983 Mar;336:433–451. doi: 10.1113/jphysiol.1983.sp014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977 Mar;69(3):325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara T., Kimizuka H., Koketsu K. An analysis of the membrane hyperpolarization during action of the sodium pump in frog's skeletal muscles. Jpn J Physiol. 1973 Apr;23(2):165–181. doi: 10.2170/jjphysiol.23.165. [DOI] [PubMed] [Google Scholar]
- Terrar D. A. Influence of SKF-525A congeners, strophanthidin and tissue-culture media on desensitization in frog skeletal muscle. Br J Pharmacol. 1974 Jun;51(2):259–268. doi: 10.1111/j.1476-5381.1974.tb09656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff S., Vyskocil F., Ward M. R. The action potential in end-plate and extrajunctional regions of rat skeletal muscle. Acta Physiol Scand. 1974 Jun;91(2):196–202. doi: 10.1111/j.1748-1716.1974.tb05676.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L. Electrogenic sodium pumping in Xenopus blastomeres: apparent pump conductance and reversal potential. Soc Gen Physiol Ser. 1984;38:345–351. [PubMed] [Google Scholar]
- Wray D. Prolonged exposure to acetylcholine: noise analysis and channel inactivation in cat tenuissimus muscle. J Physiol. 1981 Jan;310:37–56. doi: 10.1113/jphysiol.1981.sp013536. [DOI] [PMC free article] [PubMed] [Google Scholar]


