Abstract
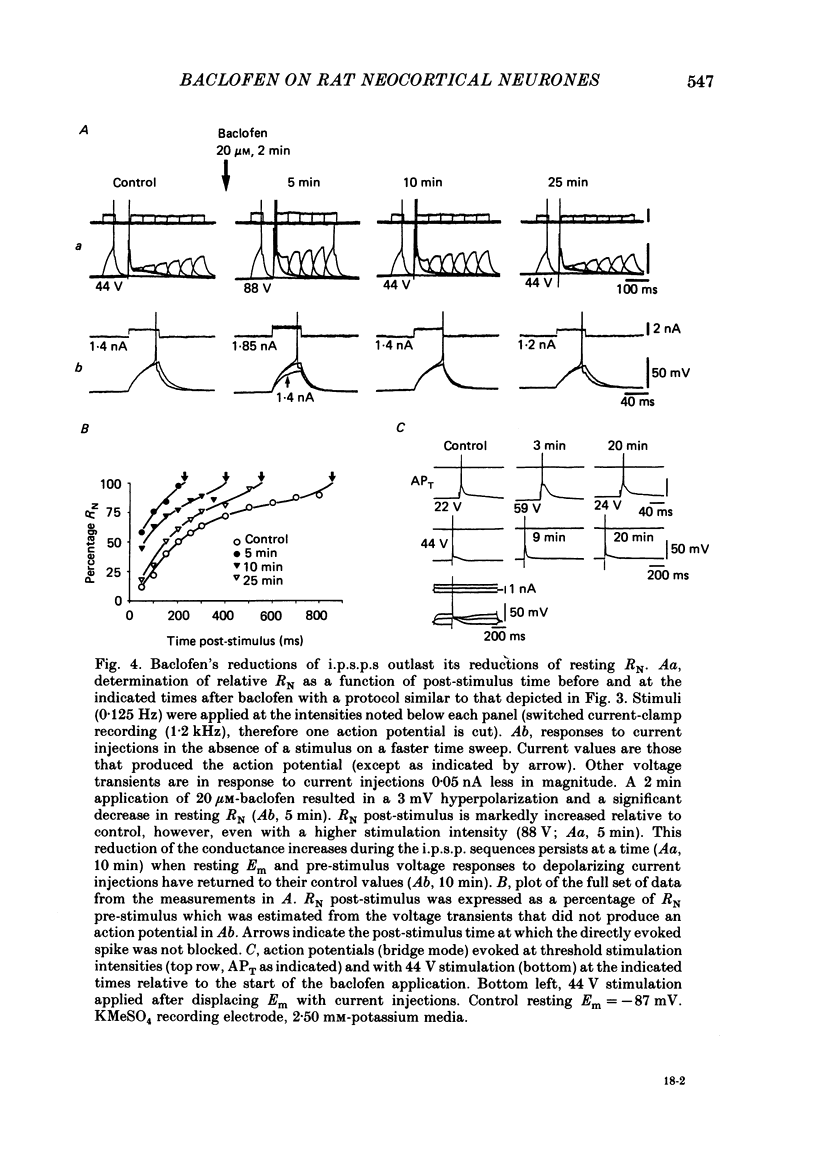
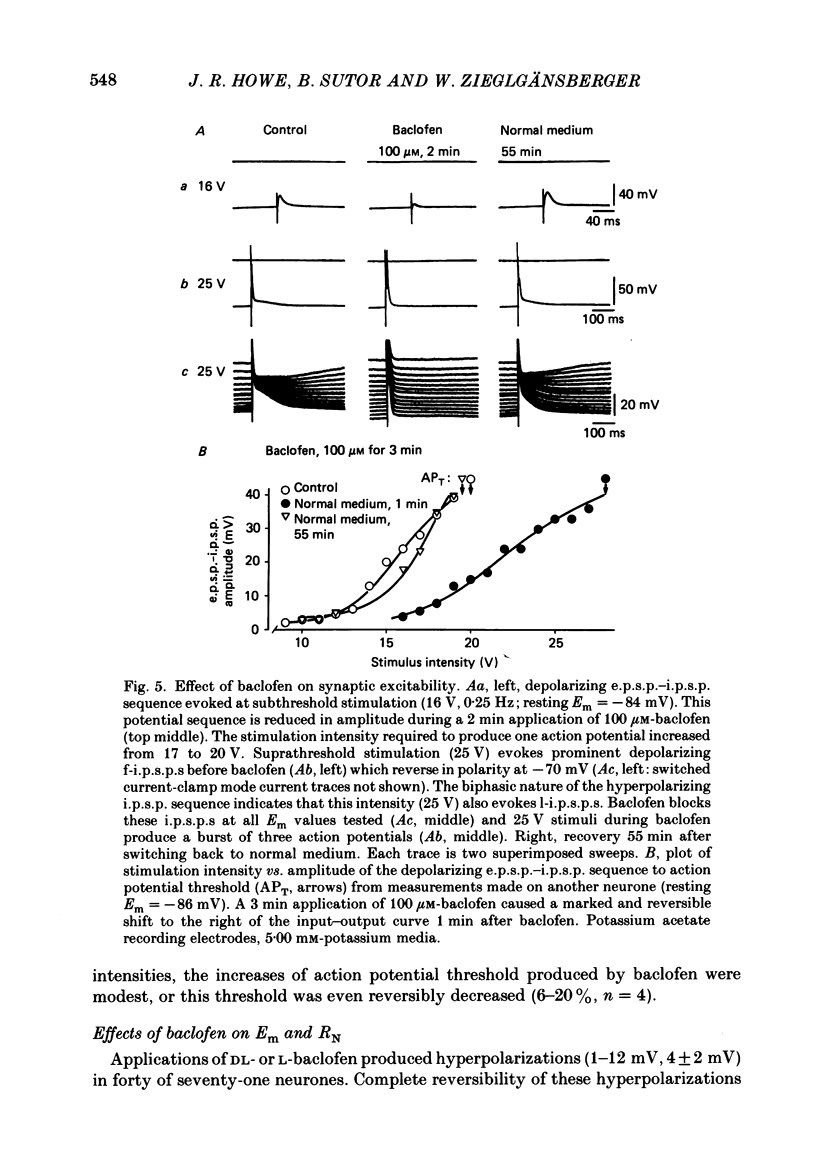
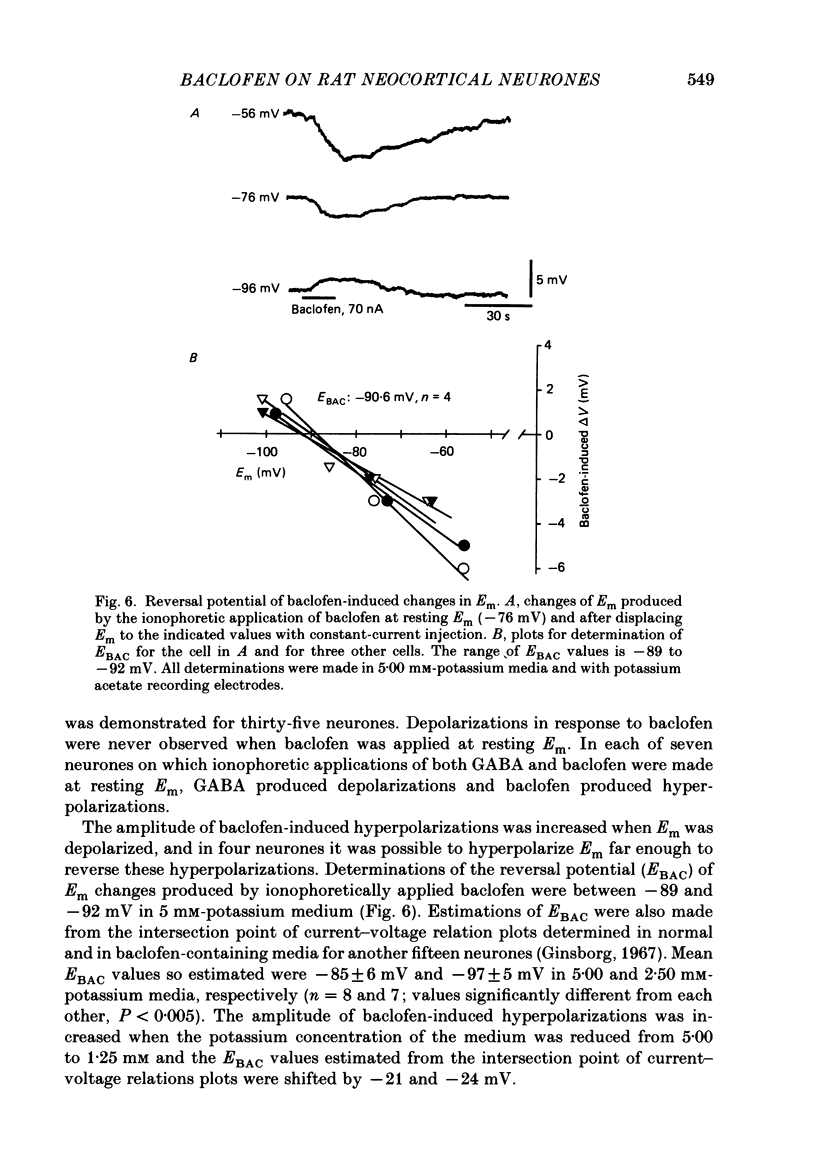
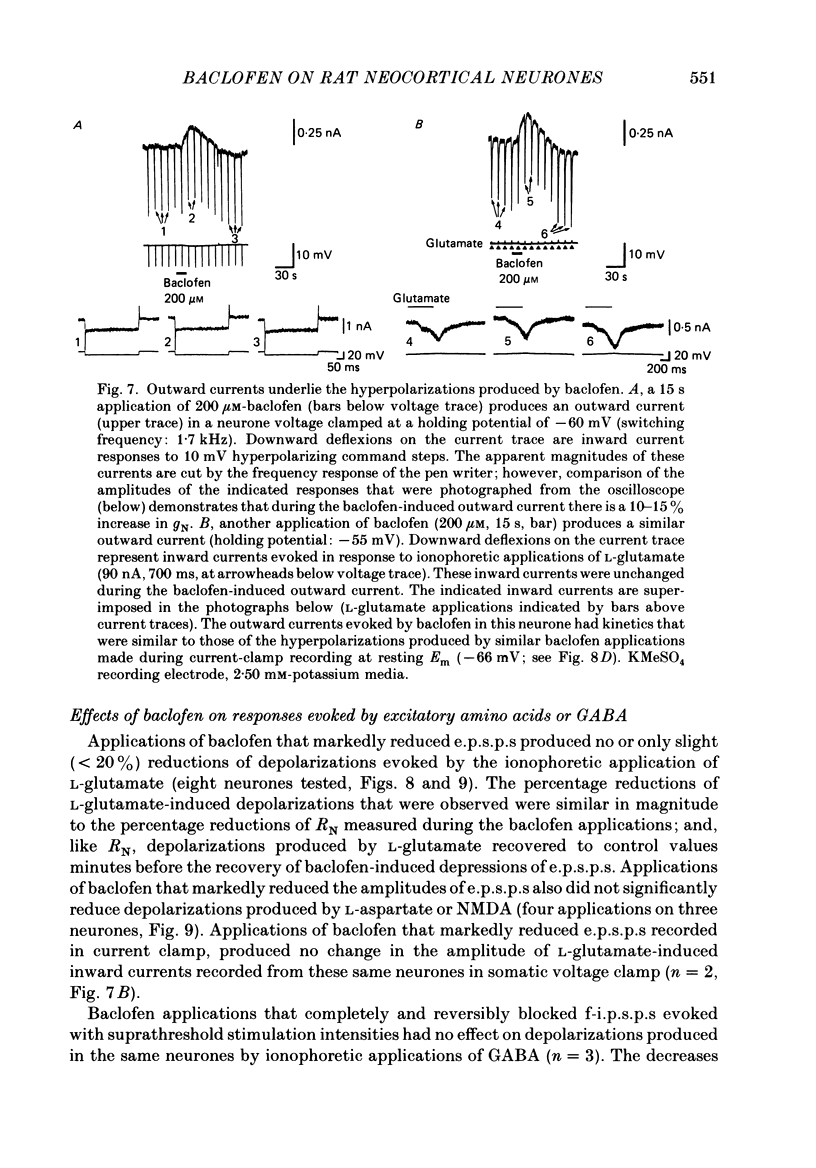
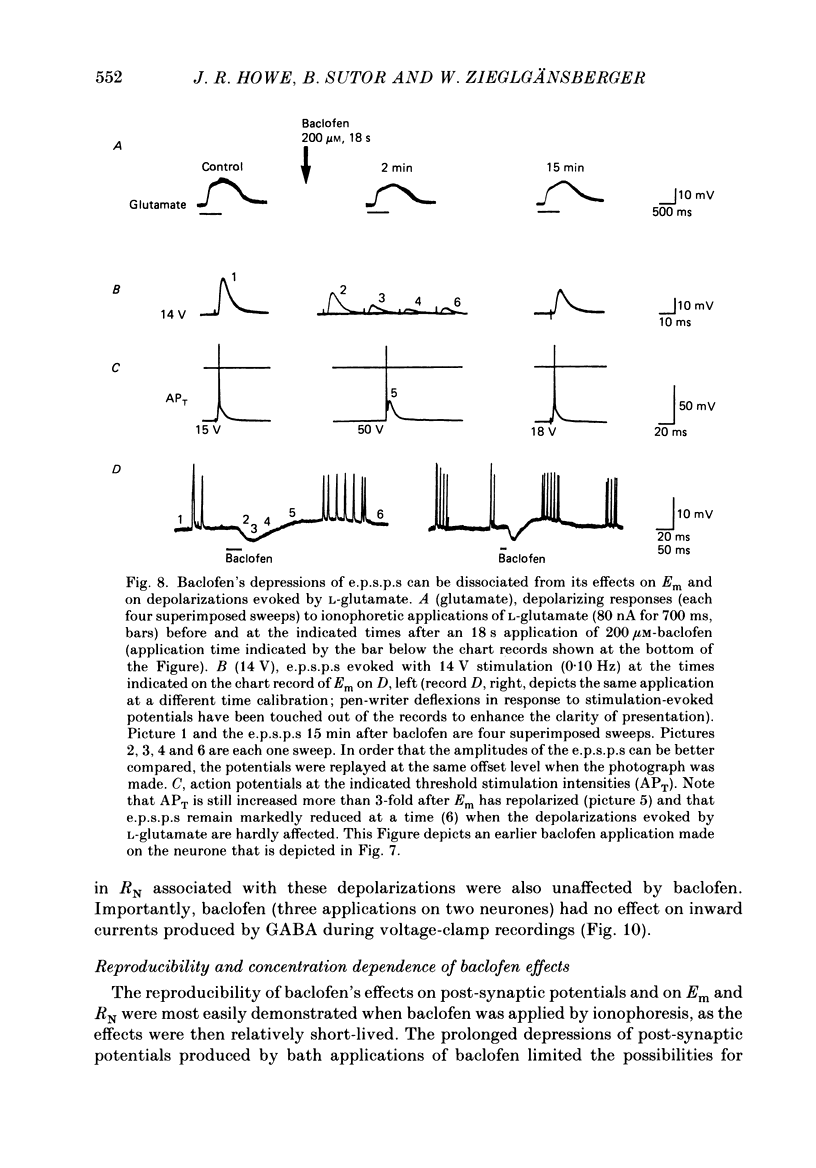
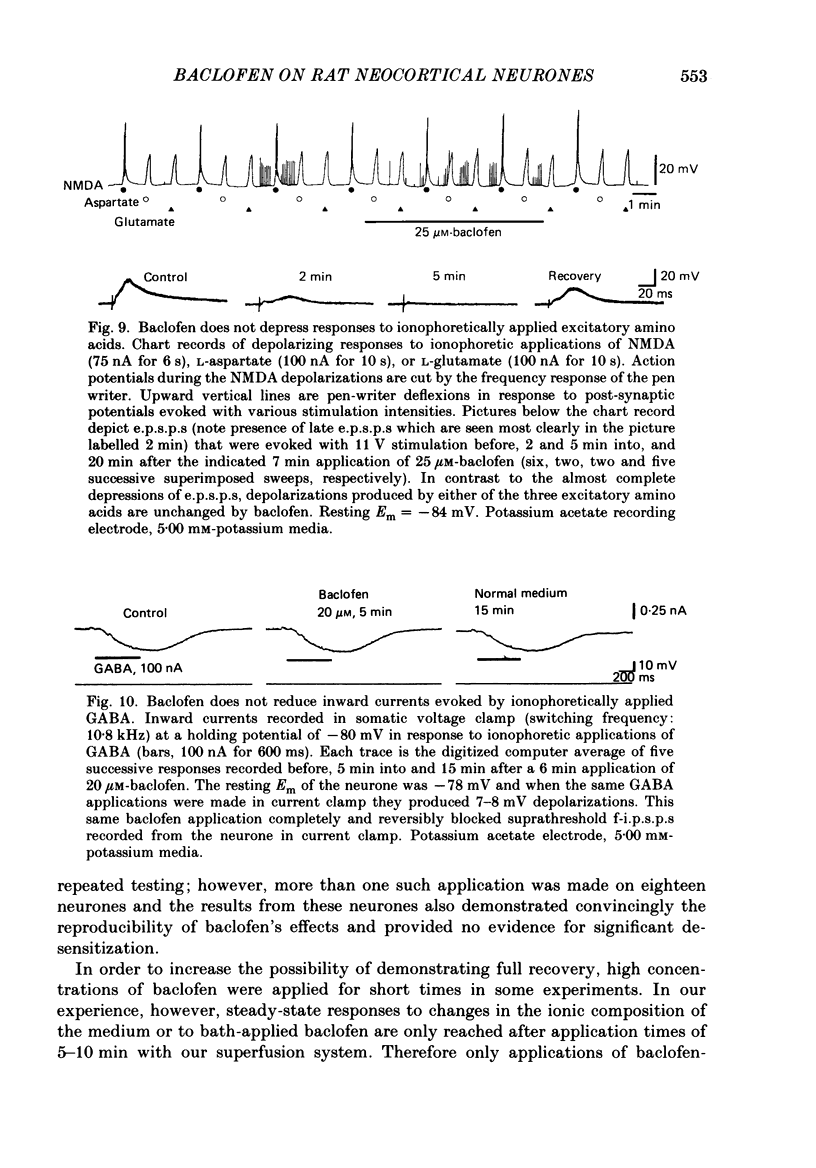
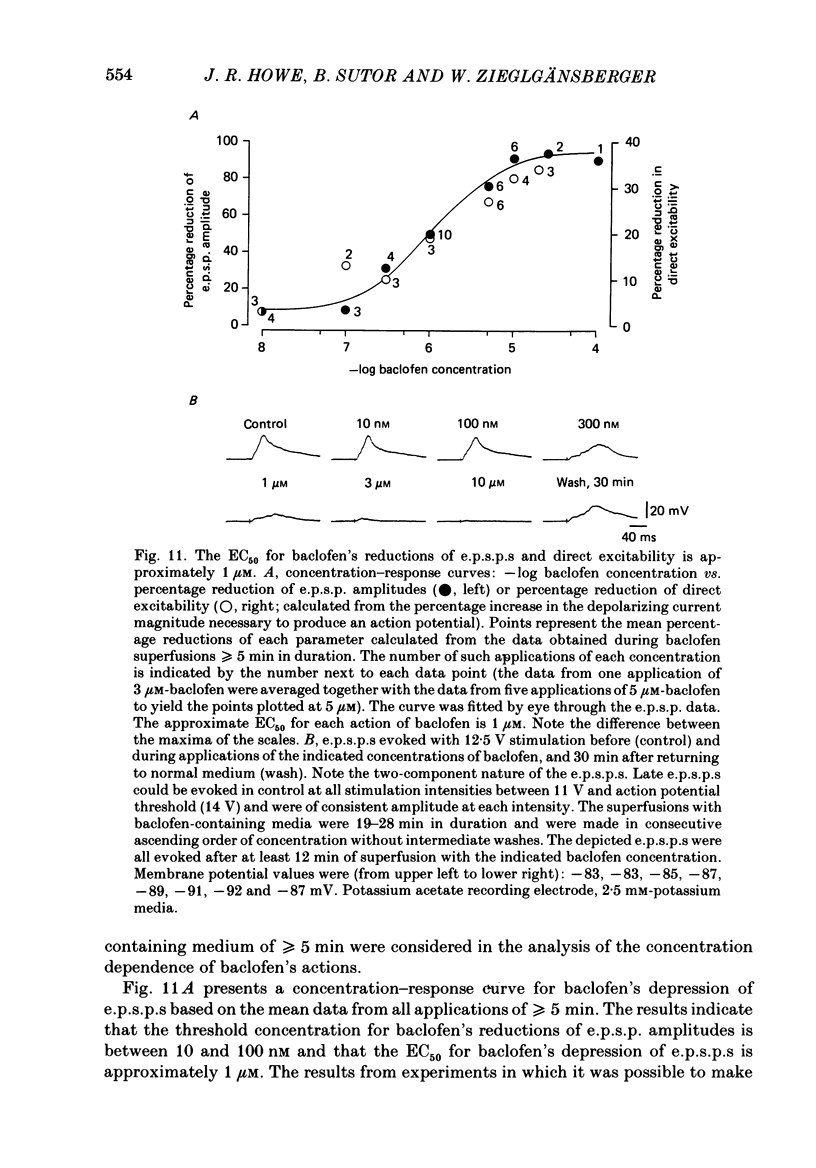
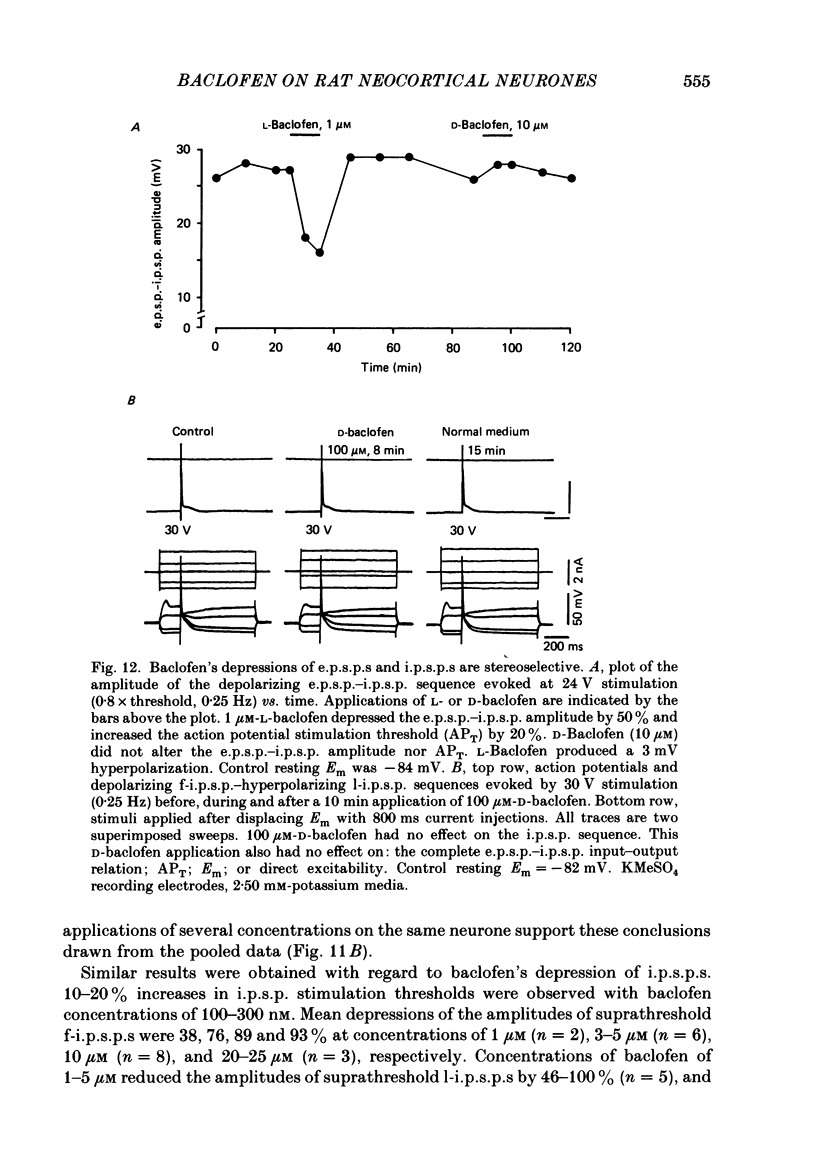
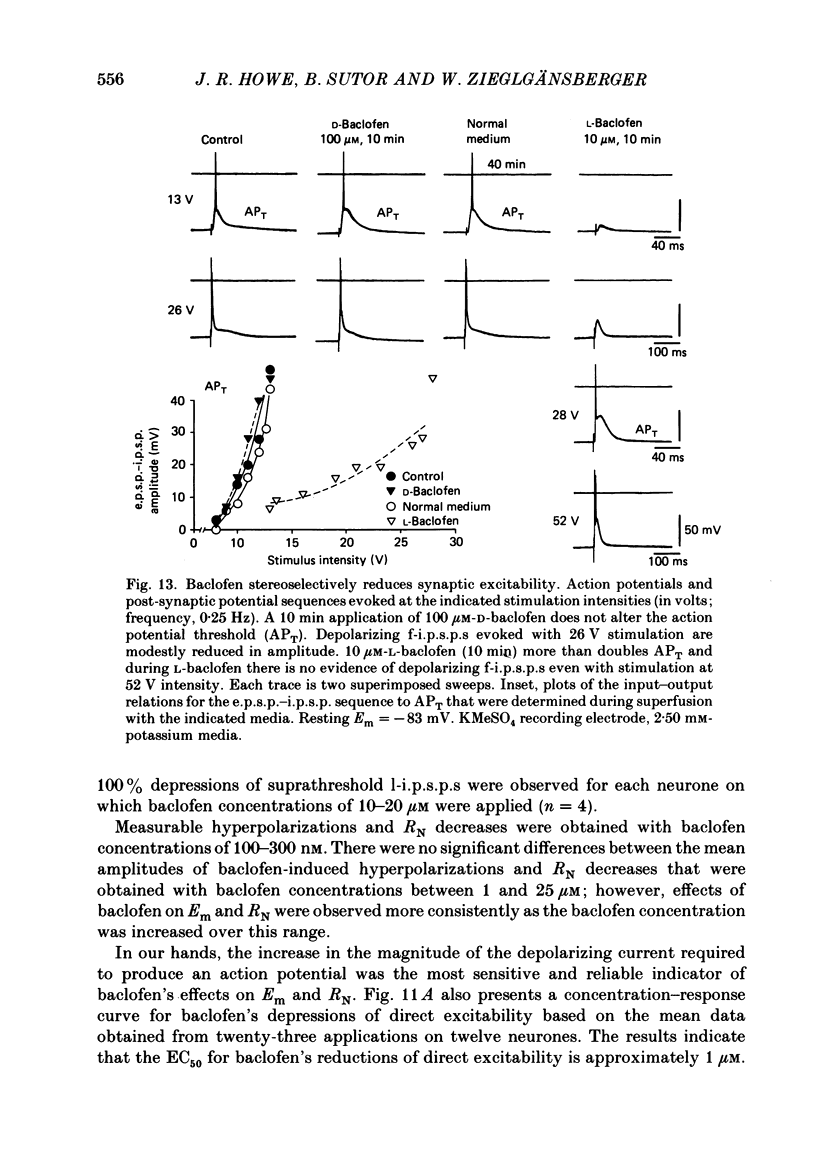
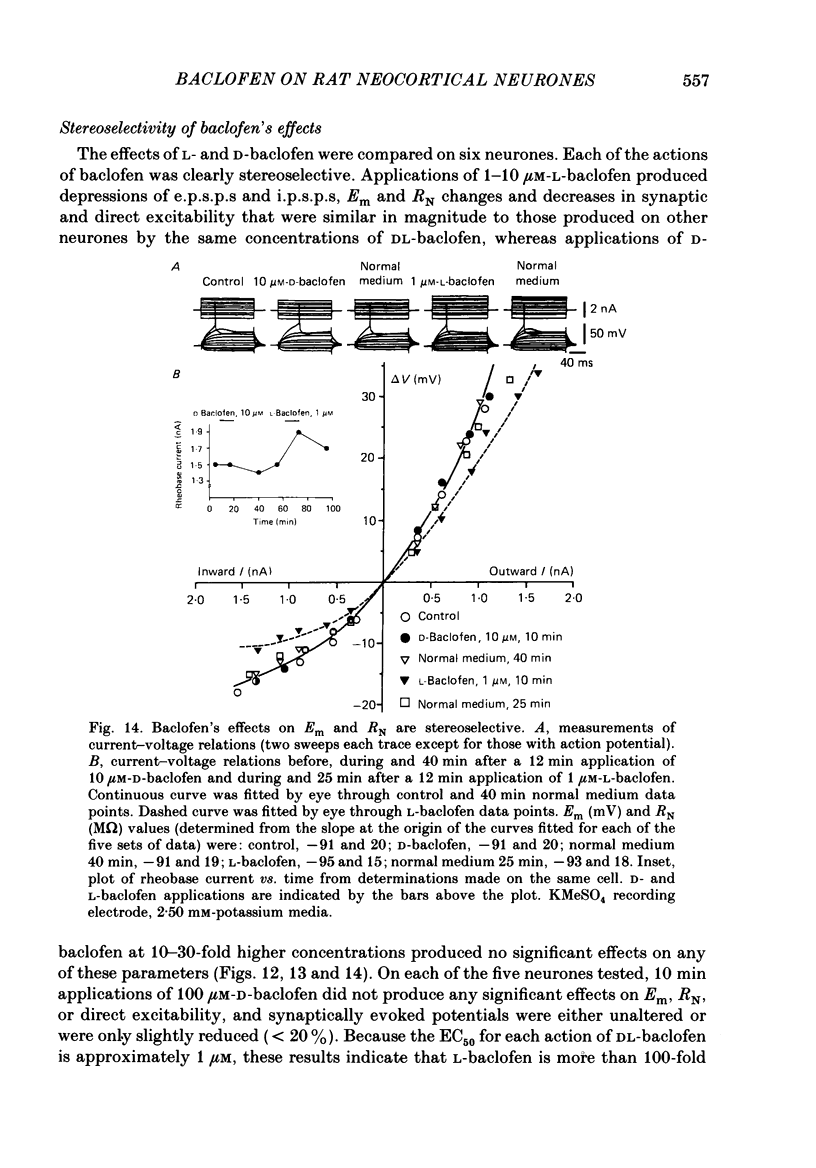
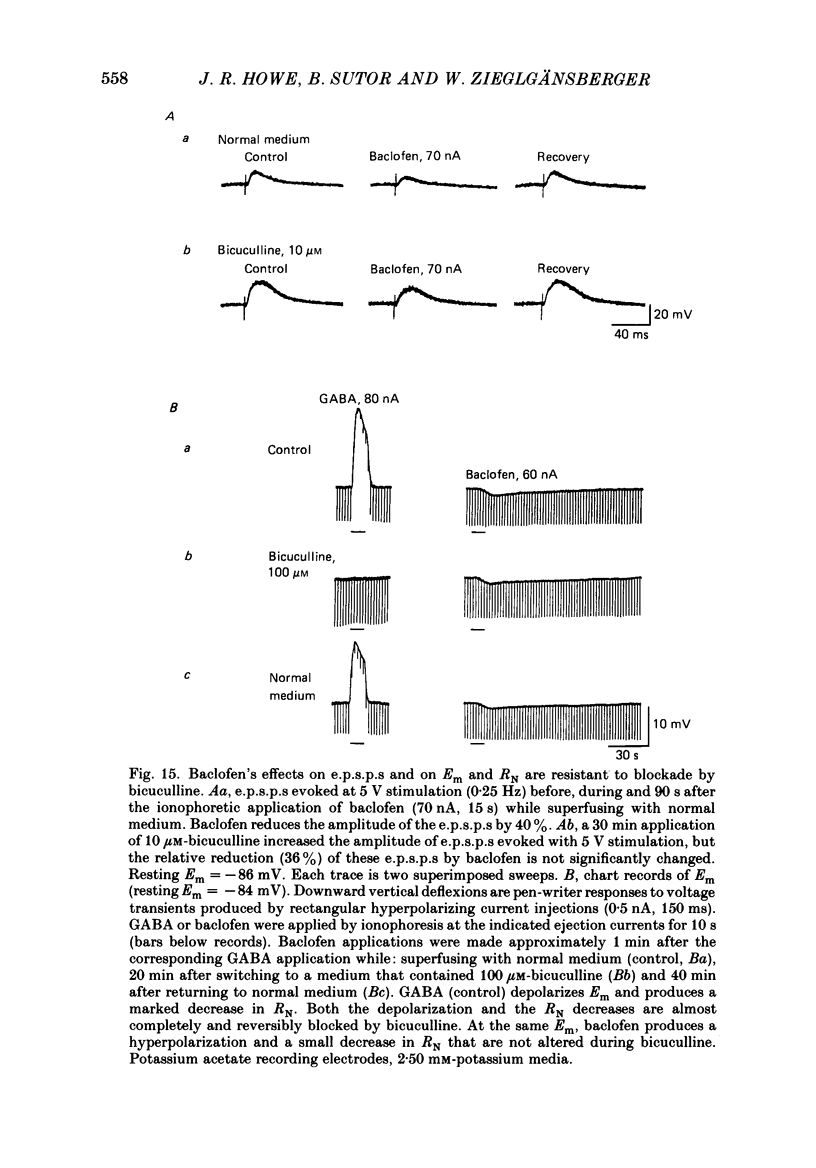
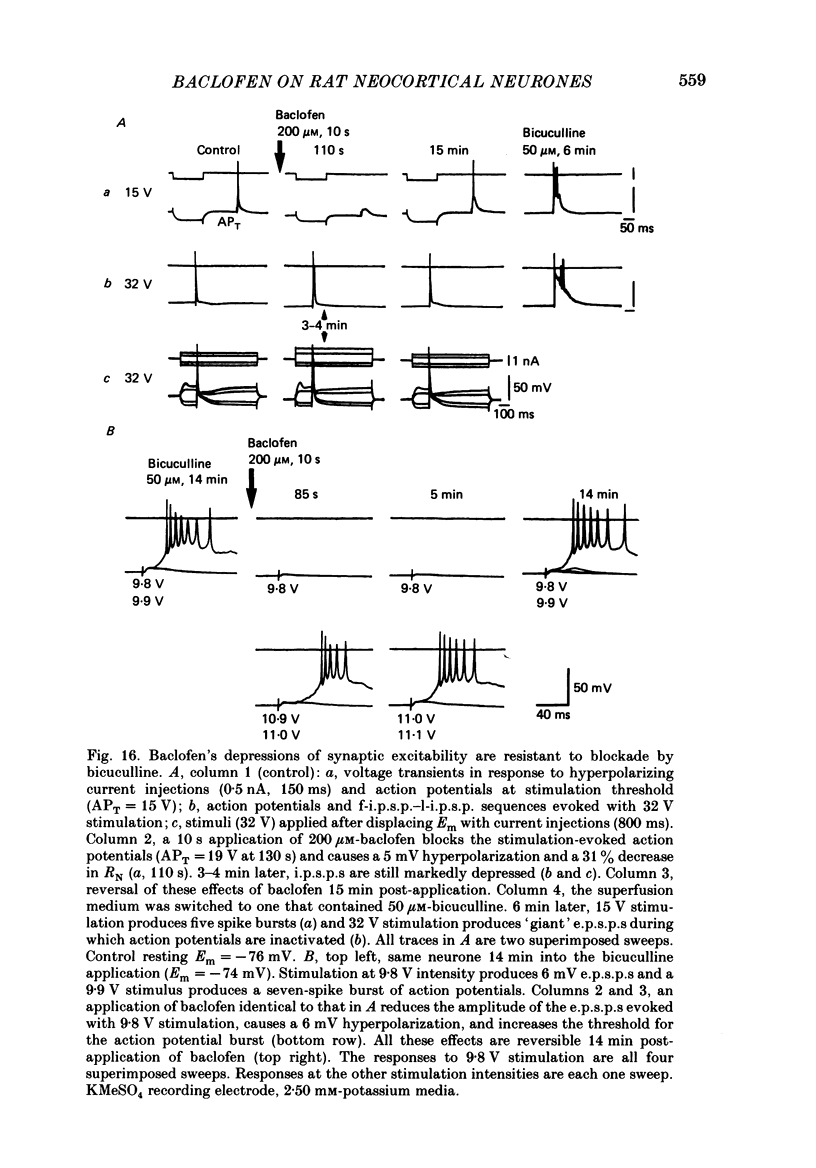
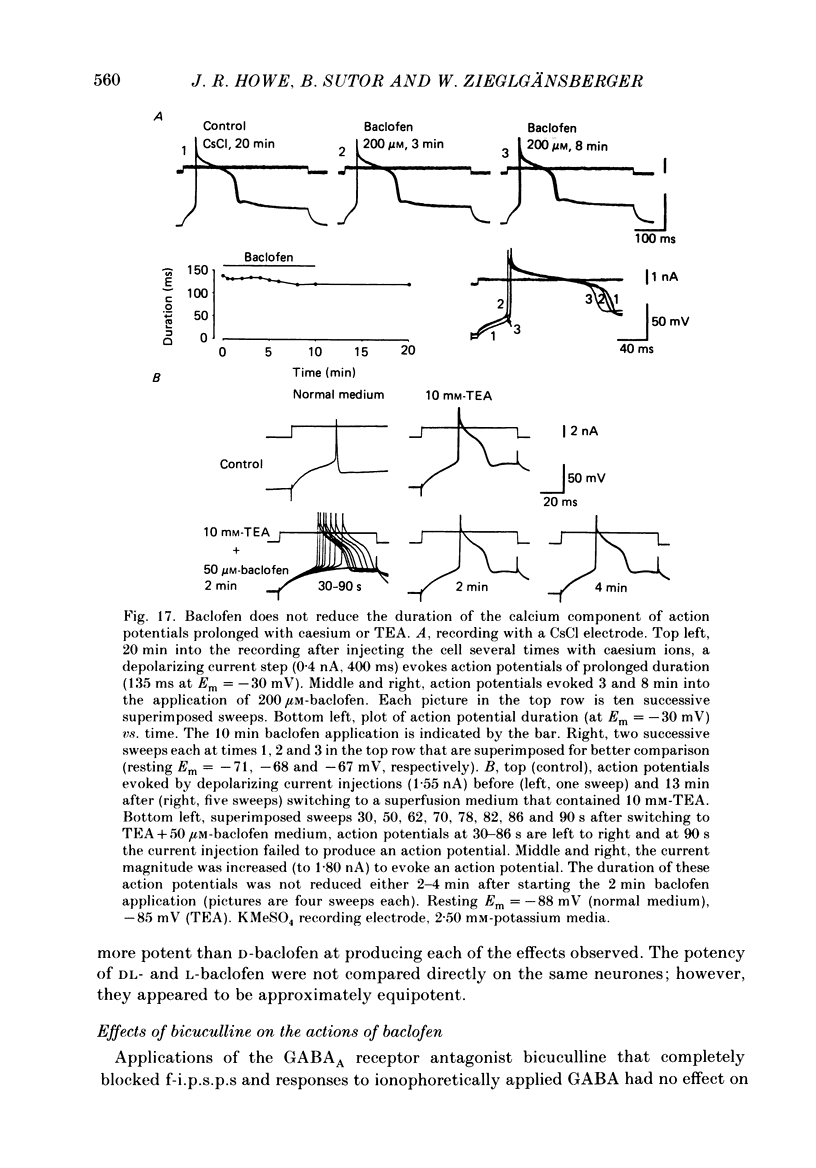
1. Intracellular recordings were obtained from neurones in layers 2 and 3 of the rat frontal neocortex in an in vitro slice preparation. Three distinct types of stimulation-evoked post-synaptic potentials were recorded in these neurones: excitatory post-synaptic potentials (e.p.s.p.s); bicuculline-sensitive, chloride-dependent inhibitory post-synaptic potentials (i.p.s.p.s) with times to peak of 20-25 ms (fast(f)-i.p.s.p.s); bicuculline-insensitive, potassium-dependent i.p.s.p.s with bicuculline-insensitive, potassium-dependent i.p.s.p.s with times to peak of 150-250 ms (long(l)-i.p.s.p.s). 2. The effects of baclofen were investigated on seventy-one neurones. Baclofen was applied by ionophoresis or pressure ejection from micropipettes or was added to the superfusion medium. 3. Baclofen depressed stimulation-evoked e.p.s.p.s in fifty-seven of the sixty neurones tested. This effect was associated with an increase in the stimulation intensity required to produce a synaptically evoked action potential for thirty-nine of forty-four neurones. 4. Baclofen depressed f-i.p.s.p.s in thirty-seven of the thirty-nine neurones tested and l-i.p.s.p.s in each one of the seventeen neurones tested. Reversal potential values for each type of i.p.s.p. were not changed by baclofen and its depressions of each were independent of membrane potential (Em). Baclofen reduced the magnitude and the duration of the conductance increases that were associated with f- and l-i.p.s.p.s. 5. Baclofen hyperpolarized forty of seventy-one neurones and produced outward currents in three of four neurones recorded in voltage clamp at holding potentials between -55 and -65 mV. These actions were associated with 10-58% reductions of neuronal input resistance (RN) and 10-20% increases in neuronal input conductance (gN), respectively. Baclofen decreased the direct excitability of twenty-three of twenty-seven neurones tested. Determinations of the reversal potential for baclofen-induced changes of Em indicate that baclofen increases the conductance of rat neocortical neurones to potassium ions. 6. The EC50 for each action of DL-baclofen was approximately 1 microM. L-Baclofen was greater than 100 times more potent than D-baclofen. 7. Concentrations of bicuculline that blocked f-i.p.s.p.s and responses to ionophoretically applied gamma-aminobutyric acid (GABA) had no effect on the depressions of e.p.s.p.s or the hyperpolarizations and decreases in RN that baclofen produced. 8. Baclofen did not reduce the duration of action potentials that were prolonged with intracellular injections of caesium ions or by superfusions with medium that contained 10 mM-tetraethylammonium (TEA).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Nadler J. V. Anticonvulsant-like actions of baclofen in the rat hippocampal slice. Br J Pharmacol. 1983 Apr;78(4):701–708. doi: 10.1111/j.1476-5381.1983.tb09423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Baclofen selectively inhibits transmission at synapses made by axons of CA3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982 Nov;223(2):291–297. [PubMed] [Google Scholar]
- Barry S. R. Baclofen has a presynaptic action at the crayfish neuromuscular junction. Brain Res. 1984 Oct 8;311(1):152–156. doi: 10.1016/0006-8993(84)91409-4. [DOI] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L. Pre- and postsynaptic effects of baclofen in the rat hippocampal slice. Brain Res. 1985 Aug 19;341(1):195–199. doi: 10.1016/0006-8993(85)91489-1. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brady R. J., Swann J. W. Postsynaptic actions of baclofen associated with its antagonism of bicuculline-induced epileptogenesis in hippocampus. Cell Mol Neurobiol. 1984 Dec;4(4):403–408. doi: 10.1007/BF00733601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. R., Simmonds M. A. Effects of baclofen on the olfactory cortex slice preparation. Neuropharmacology. 1982 Apr;21(4):371–373. doi: 10.1016/0028-3908(82)90103-4. [DOI] [PubMed] [Google Scholar]
- Capek R., Esplin B. Baclofen-induced decrease of excitability of primary afferents and depression of monosynaptic transmission in cat spinal cord. Can J Physiol Pharmacol. 1982 Feb;60(2):160–166. doi: 10.1139/y82-026. [DOI] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Inhibition of calcium spikes and transmitter release by gamma-aminobutyric acid in the guinea-pig myenteric plexus. Br J Pharmacol. 1984 May;82(1):101–105. doi: 10.1111/j.1476-5381.1984.tb16446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins G. G., Anson J., Kelly E. P. Baclofen: effects on evoked field potentials and amino acid neurotransmitter release in the rat olfactory cortex slice. Brain Res. 1982 Apr 29;238(2):371–383. doi: 10.1016/0006-8993(82)90111-1. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Sears E. S. The effects of Lioresal on synaptic activity in the isolated spinal cord. Neurology. 1974 Oct;24(10):957–963. doi: 10.1212/wnl.24.10.957. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Gehlert D. R., Yamamura H. I., Wamsley J. K. gamma-Aminobutyric acidB receptors in the rat brain: quantitative autoradiographic localization using [3H](-)-baclofen. Neurosci Lett. 1985 May 14;56(2):183–188. doi: 10.1016/0304-3940(85)90126-0. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Greene R. W., Olpe H. R. Stereoselectivity of L-baclofen in hippocampal slices of the rat. Neurosci Lett. 1985 Mar 22;55(1):1–4. doi: 10.1016/0304-3940(85)90302-7. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Hamon B., Konnerth A. GABA and baclofen reduce changes in extracellular free calcium in area CA1 of rat hippocampal slices. Neurosci Lett. 1984 Jun 29;47(3):295–300. doi: 10.1016/0304-3940(84)90529-9. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Baclofen activates voltage-dependent and 4-aminopyridine sensitive K+ conductance in guinea-pig hippocampal pyramidal cells maintained in vitro. Br J Pharmacol. 1985 Apr;84(4):833–841. doi: 10.1111/j.1476-5381.1985.tb17377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Characterization of pre- and postsynaptic actions of (-)-baclofen in the guinea-pig hippocampus in vitro. Br J Pharmacol. 1985 Apr;84(4):843–851. doi: 10.1111/j.1476-5381.1985.tb17378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Hailstone M. H., Freeman C. G. Baclofen: stereoselective inhibition of excitant amino acid release. J Pharm Pharmacol. 1980 Mar;32(3):230–231. doi: 10.1111/j.2042-7158.1980.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Karbon E. W., Duman R., Enna S. J. Biochemical identification of multiple GABAB binding sites: association with noradrenergic terminals in rat forebrain. Brain Res. 1983 Sep 12;274(2):393–396. doi: 10.1016/0006-8993(83)90725-4. [DOI] [PubMed] [Google Scholar]
- Knutsson E., Lindblom U., Mårtensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci. 1974 Nov;23(3):473–484. doi: 10.1016/0022-510x(74)90163-4. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Baudry M., Fagni L., Lynch G. The blocking action of baclofen on excitatory transmission in the rat hippocampal slice. J Neurosci. 1982 Jun;2(6):698–703. doi: 10.1523/JNEUROSCI.02-06-00698.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Pinnock R. D. Hyperpolarizing action of baclofen on neurons in the rat substantia nigra slice. Brain Res. 1984 Nov 26;322(2):337–340. doi: 10.1016/0006-8993(84)90129-x. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release and metabolism in slices of guinea pig cerebral cortex. J Neurochem. 1979 Jan;32(1):103–109. doi: 10.1111/j.1471-4159.1979.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Schlicker E., Classen K., Göthert M. GABAB receptor-mediated inhibition of serotonin release in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(2):99–105. doi: 10.1007/BF00517304. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Baclofen blocks postsynaptic inhibition but not the effect of muscimol in the olfactory cortex. Br J Pharmacol. 1983 Jan;78(1):79–84. doi: 10.1111/j.1476-5381.1983.tb09365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Further studies on the action of baclofen on neurons of the dorsolateral septal nucleus of the rat, in vitro. Brain Res. 1985 Dec 9;358(1-2):360–363. doi: 10.1016/0006-8993(85)90984-9. [DOI] [PubMed] [Google Scholar]
- Swahn C. G., Beving H., Sedvall G. Mass fragmentographic determination of 4-amino-3-p-chlorophenylbutyric acid (baclofen) in cerebrospinal fluid and serum. J Chromatogr. 1979 Mar 1;162(3):433–438. doi: 10.1016/s0378-4347(00)81532-7. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


