Abstract
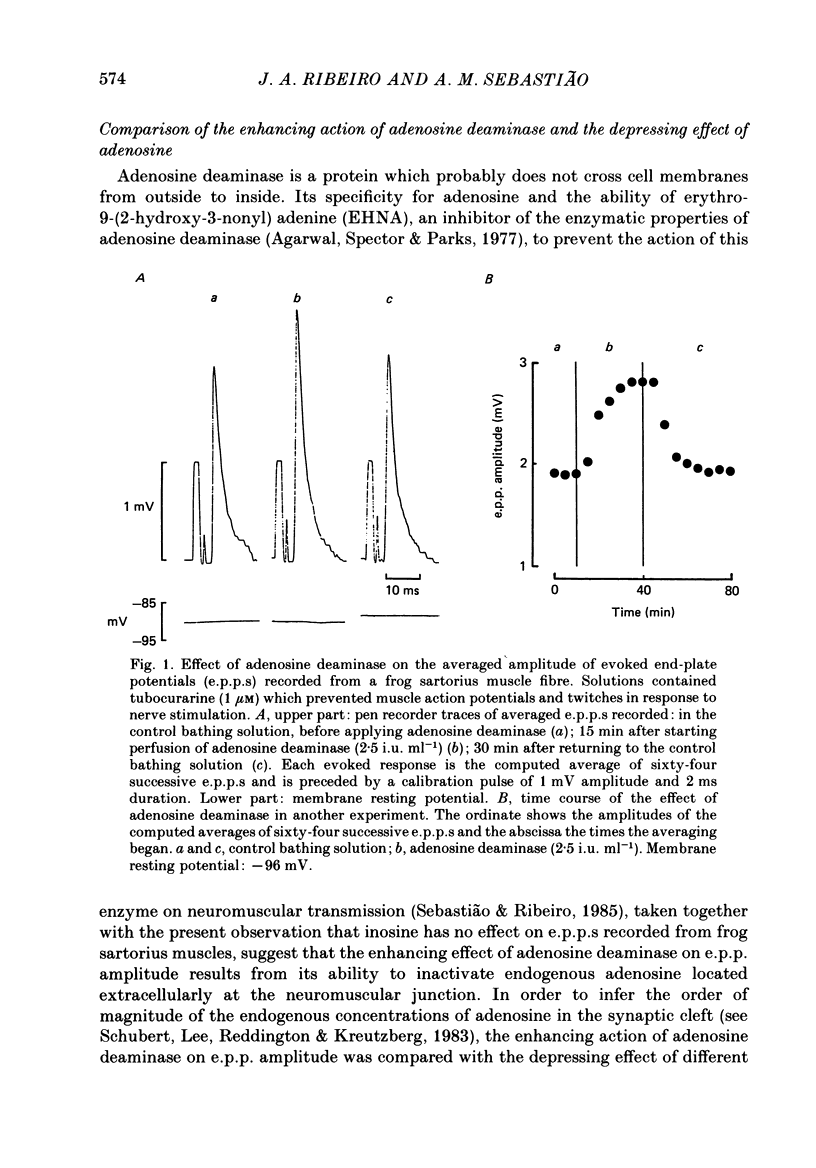
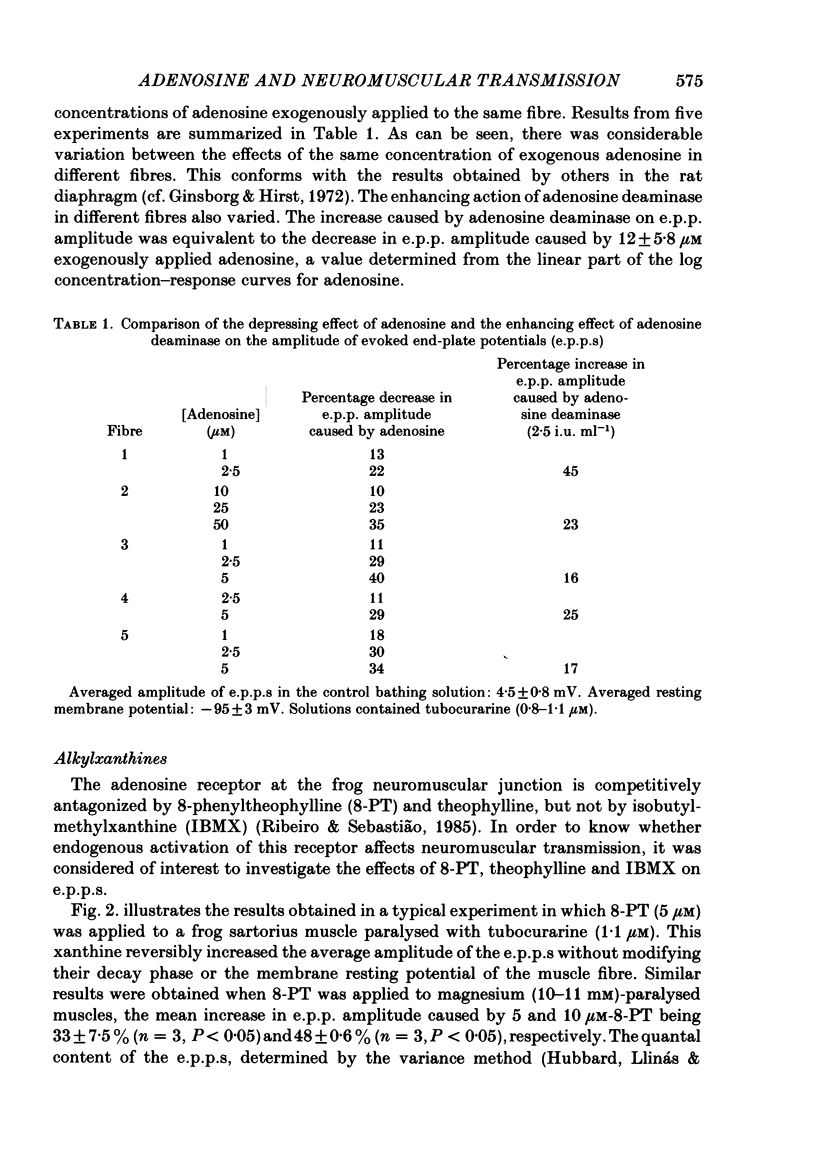
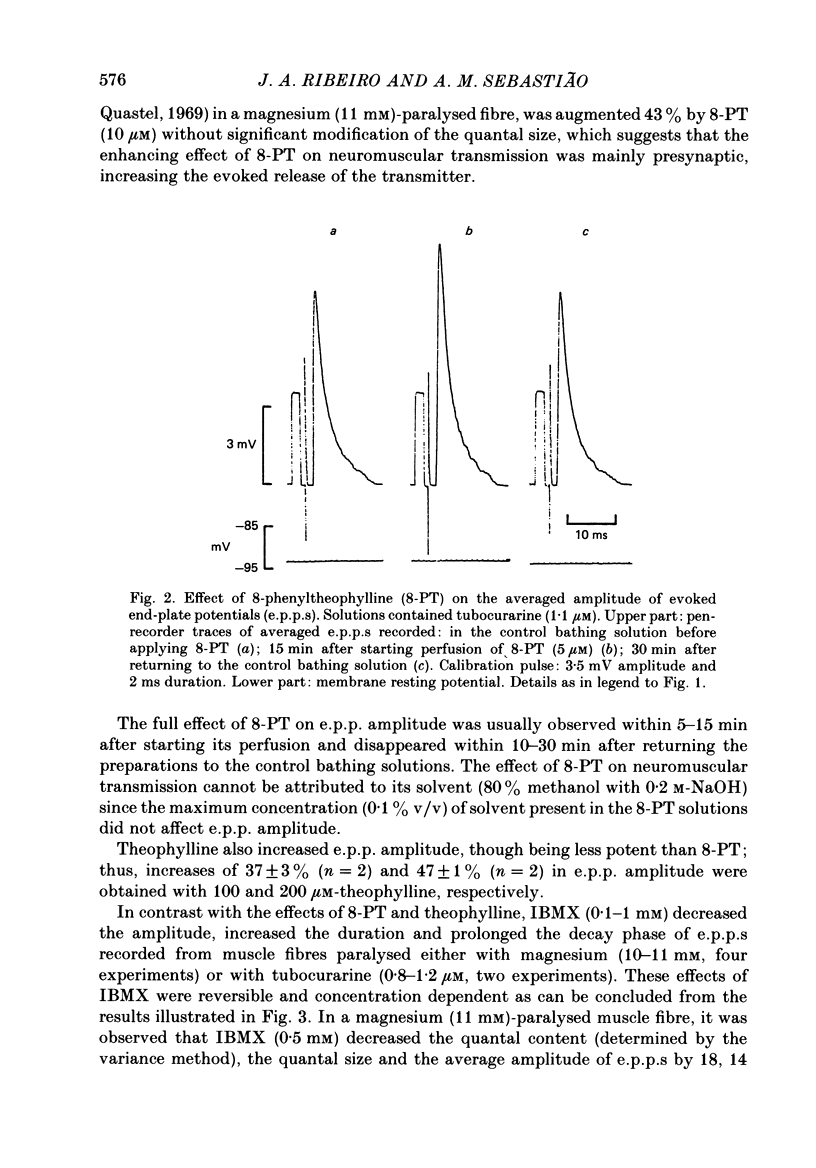
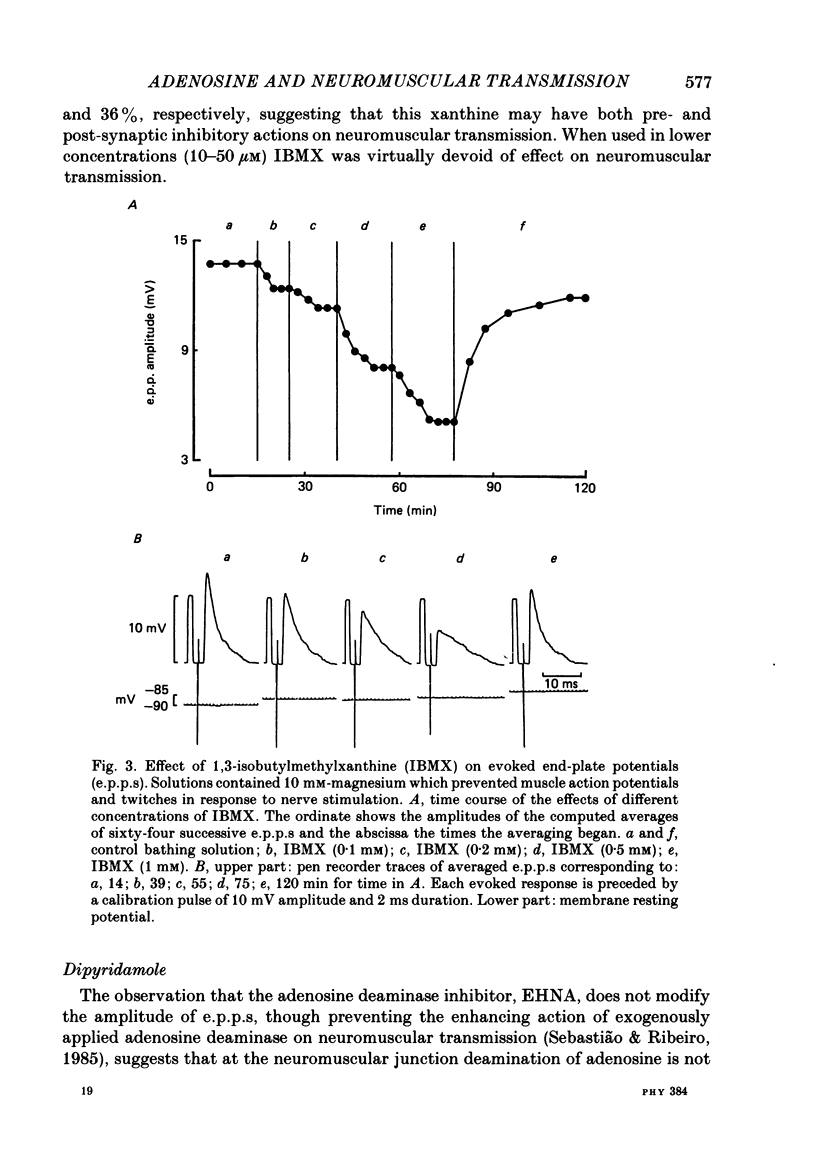
1. The effects of adenosine deaminase, inosine, alkylxanthines (8-phenyltheophylline (8-PT), theophylline and isobutylmethylxanthine (IBMX], dipyridamole, alpha, beta-methylene ADP (AOPCP) and ATP analogues (alpha, beta-methylene ATP and beta, gamma-methylene ATP) on evoked end-plate potentials (e.p.p.s) were investigated in innervated sartorius muscles of the frog, in which twitches had been prevented with tubocurarine. The effects of 8-PT and IBMX on the amplitude and quantal content of e.p.p.s were also investigated in innervated sartorius muscles of the frog, in which twitches had been prevented with high-magnesium solutions. 2. Adenosine deaminase reversibly increased the amplitude of e.p.p.s and prevented the reduction caused by exogenously applied adenosine on e.p.p. amplitude. The increase caused by adenosine deaminase was equivalent to the decrease caused by 12 +/- 5.8 microM-adenosine on e.p.p. amplitude. 3. Inosine, the product of adenosine deamination, was virtually devoid of effect on e.p.p.s. 4. The adenosine receptor antagonists at the frog neuromuscular junction, 8-PT and theophylline, increased in a concentration-dependent manner the amplitude of e.p.p.s in the presence of tubocurarine. 8-PT increased the amplitude and quantal content of e.p.p.s in the presence of high magnesium. IBMX, which does not behave as an adenosine receptor antagonist at the frog neuromuscular junction, decreased the amplitude of e.p.p.s in the presence of tubocurarine or high-magnesium solutions. 5. Dipyridamole, an adenosine uptake blocker, decreased the amplitude of e.p.p.s, and in a concentration that did not affect neuromuscular transmission potentiated the depressing effect of adenosine, but not that of 2-chloroadenosine, on the amplitude of e.p.p.s. 6. AOPCP, an inhibitor of 5'-nucleotidase, increased the amplitude of e.p.p.s and markedly attenuated the depressing effect of ATP, but not that of adenosine, on e.p.p. amplitude. 7. The ATP analogue, alpha, beta-methylene ATP, which is not a substrate for 5'-nucleotidase, was virtually devoid of effect on e.p.p.s. beta, gamma-Methylene ATP, which can be a substrate for 5'-nucleotidase, mimicked the depressing effect of ATP on e.p.p. amplitude, an effect which was also reduced by AOPCP. 8. It is concluded that in conditions in which the initial quantal content is assumed to be normal (1) endogenous adenosine depresses neuromuscular transmission, (2) at the neuromuscular junction adenosine is inactivated through a dipyridamole-sensitive uptake process, and (3) released adenine nucleotides might contribute to the pool of endogenous adenosine which modulates neuromuscular transmission.
Full text
PDF


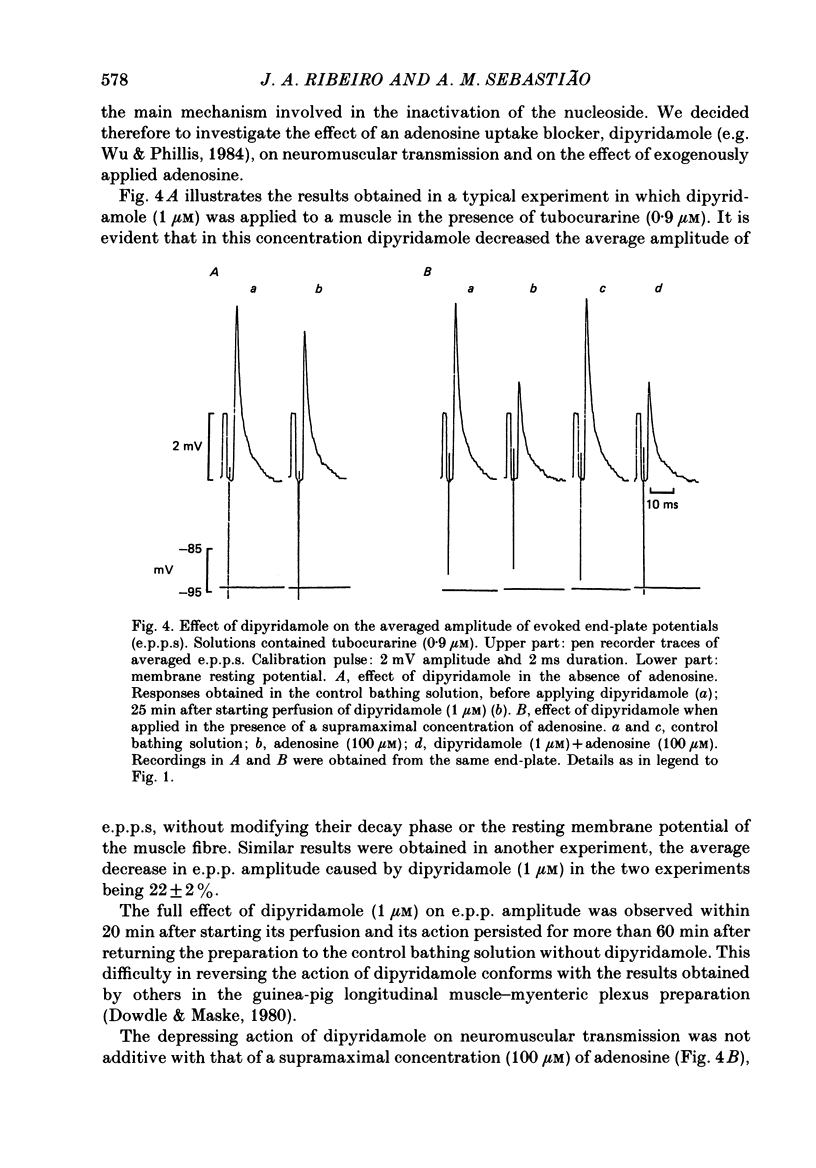
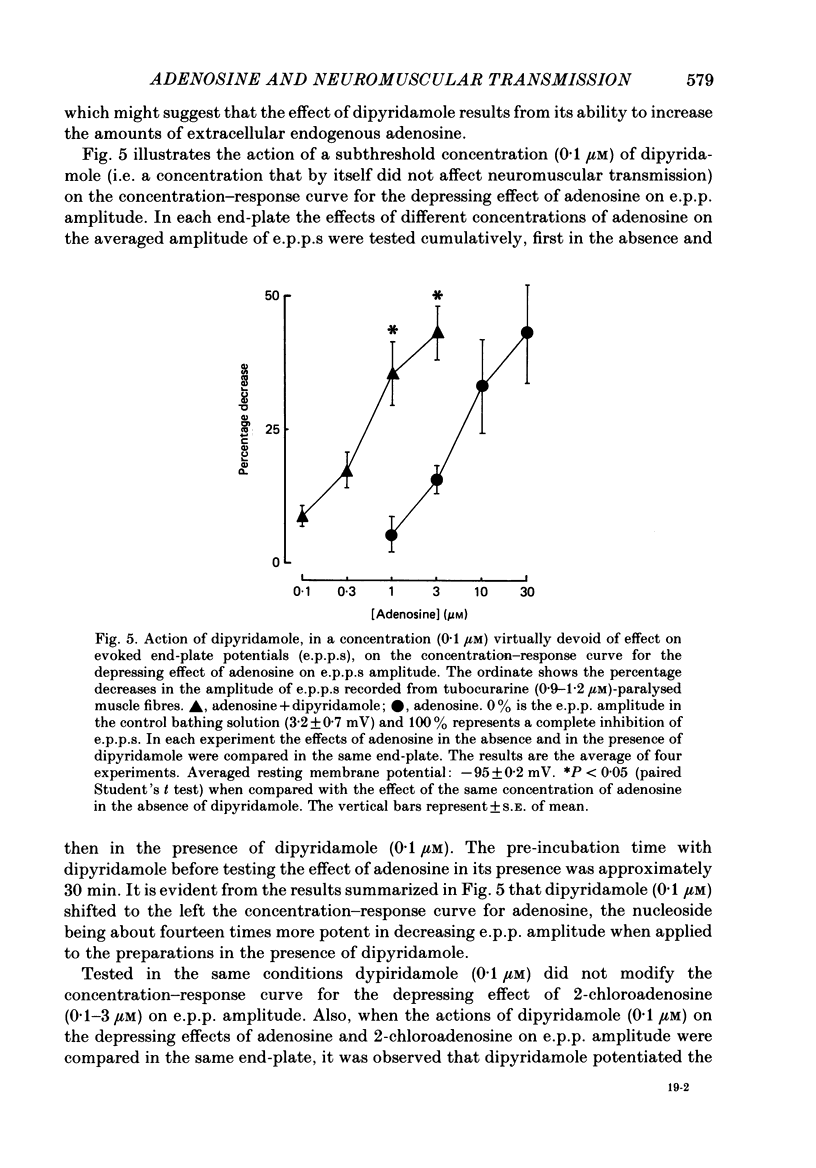

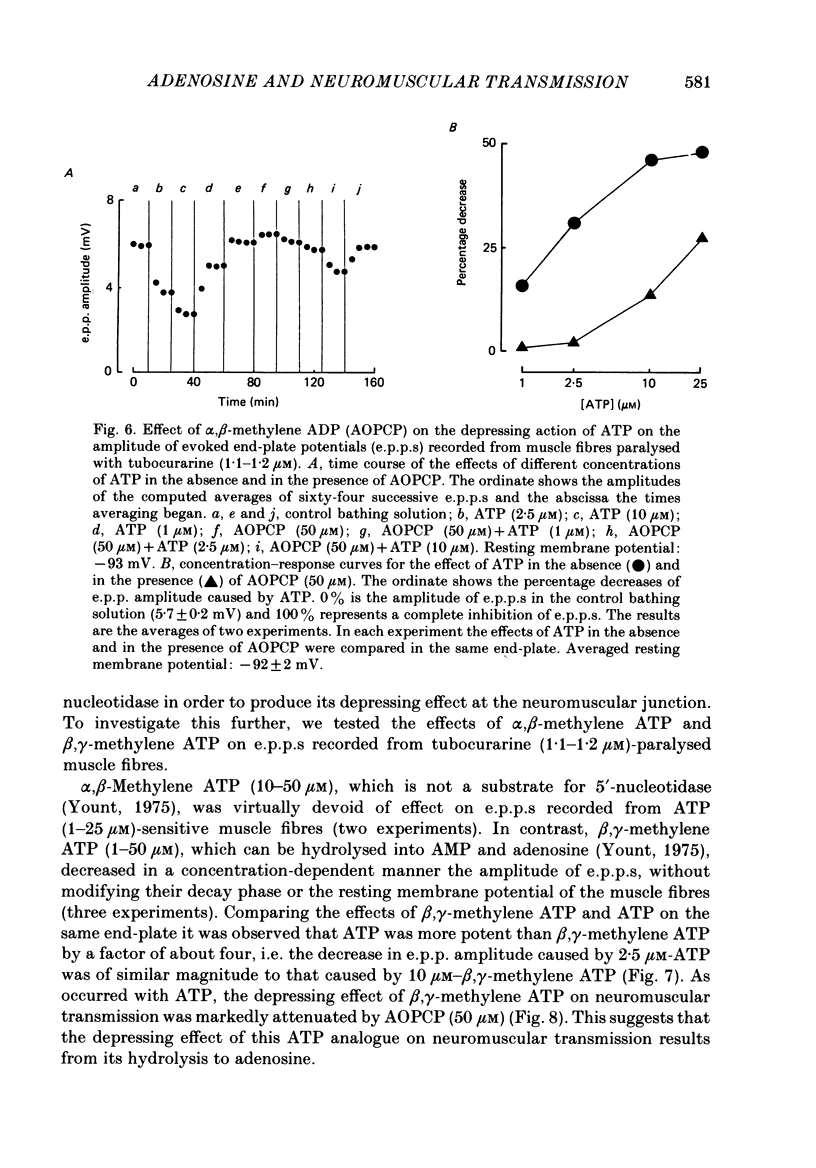
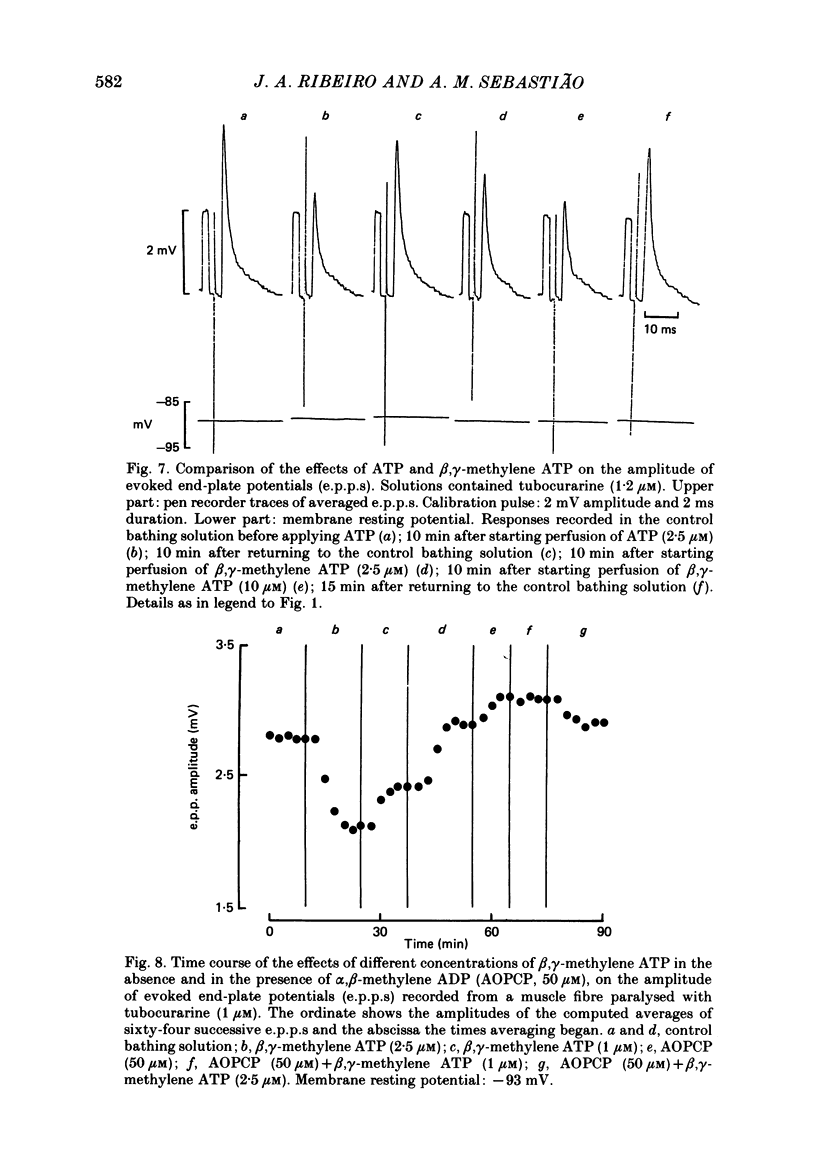



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Spector T., Parks R. E., Jr Tight-binding inhibitors--IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977 Mar 1;26(5):359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. Preparation and properties of 5'-nucleotidase from smooth muscle of small intestine. J Biol Chem. 1970 Dec 10;245(23):6274–6280. [PubMed] [Google Scholar]
- Dowdle E. B., Maske R. The effects of dipyridamole on the guinea-pig ileal longitudinal muscle-myenteric plexus preparation. Br J Pharmacol. 1980;71(1):235–244. doi: 10.1111/j.1476-5381.1980.tb10931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Hoffer B. J. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980 May;69(1):59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Jonzon B., Lindgren E., Lindström K. Adenosine receptors mediating cyclic AMP production in the rat hippocampus. J Neurochem. 1982 Jul;39(1):165–175. doi: 10.1111/j.1471-4159.1982.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol. 1972 Aug;224(3):629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Singer J. J. Evidence for a role of cyclic AMP in neuromuscular transmission. Proc Natl Acad Sci U S A. 1969 Sep;64(1):134–141. doi: 10.1073/pnas.64.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackisch R., Strittmatter H., Kasakov L., Hertting G. Endogenous adenosine as a modulator of hippocampal acetylcholine release. Naunyn Schmiedebergs Arch Pharmacol. 1984 Oct;327(4):319–325. doi: 10.1007/BF00506243. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Martin B. W., Ng A. S. 2-Chloroadenosine, a permeant for the nucleoside transporter. Biochem Pharmacol. 1985 Sep 15;34(18):3237–3241. doi: 10.1016/0006-2952(85)90340-5. [DOI] [PubMed] [Google Scholar]
- Keller F., Zimmermann H. Ecto-adenosine triphosphatase activity at the cholinergic nerve endings of the Torpedo electric organ. Life Sci. 1983 Dec 26;33(26):2635–2641. doi: 10.1016/0024-3205(83)90347-8. [DOI] [PubMed] [Google Scholar]
- Nagy A., Shuster T. A., Rosenberg M. D. Adenosine triphosphatase activity at the external surface of chicken brain synaptosomes. J Neurochem. 1983 Jan;40(1):226–234. doi: 10.1111/j.1471-4159.1983.tb12675.x. [DOI] [PubMed] [Google Scholar]
- Nimit Y., Skolnick P., Daly J. W. Adenosine and cyclic AMP in rat cerebral cortical slices: effects of adenosine uptake inhibitors and adenosine deaminase inhibitors. J Neurochem. 1981 Mar;36(3):908–912. doi: 10.1111/j.1471-4159.1981.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. On the type of receptor involved in the inhibitory action of adenosine at the neuromuscular junction. Br J Pharmacol. 1985 Apr;84(4):911–918. doi: 10.1111/j.1476-5381.1985.tb17385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. A., Walker J. The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol. 1975 Jun;54(2):213–218. doi: 10.1111/j.1476-5381.1975.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastião A. M., Ribeiro J. A. Enhancement of transmission at the frog neuromuscular junction by adenosine deaminase: evidence for an inhibitory role of endogenous adenosine on neuromuscular transmission. Neurosci Lett. 1985 Dec 4;62(2):267–270. doi: 10.1016/0304-3940(85)90366-0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Wilson D. F. The effects of dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline and aminophylline on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1974 Feb;188(2):447–452. [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Nye M. J. Alkylxanthines as adenosine receptor antagonists and membrane phosphodiesterase inhibitors in central nervous tissue: evaluation of structure-activity relationships. Life Sci. 1982 Dec 20;31(25):2857–2867. doi: 10.1016/0024-3205(82)90676-2. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]


