Abstract
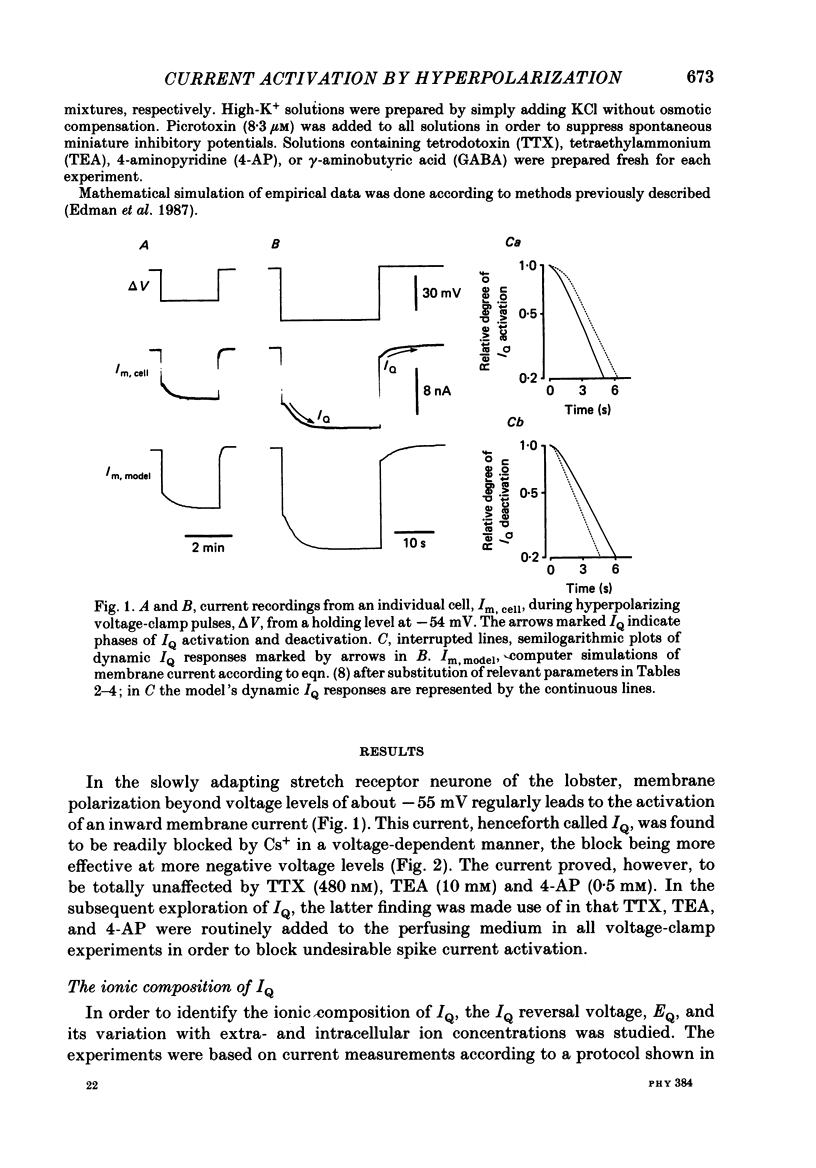
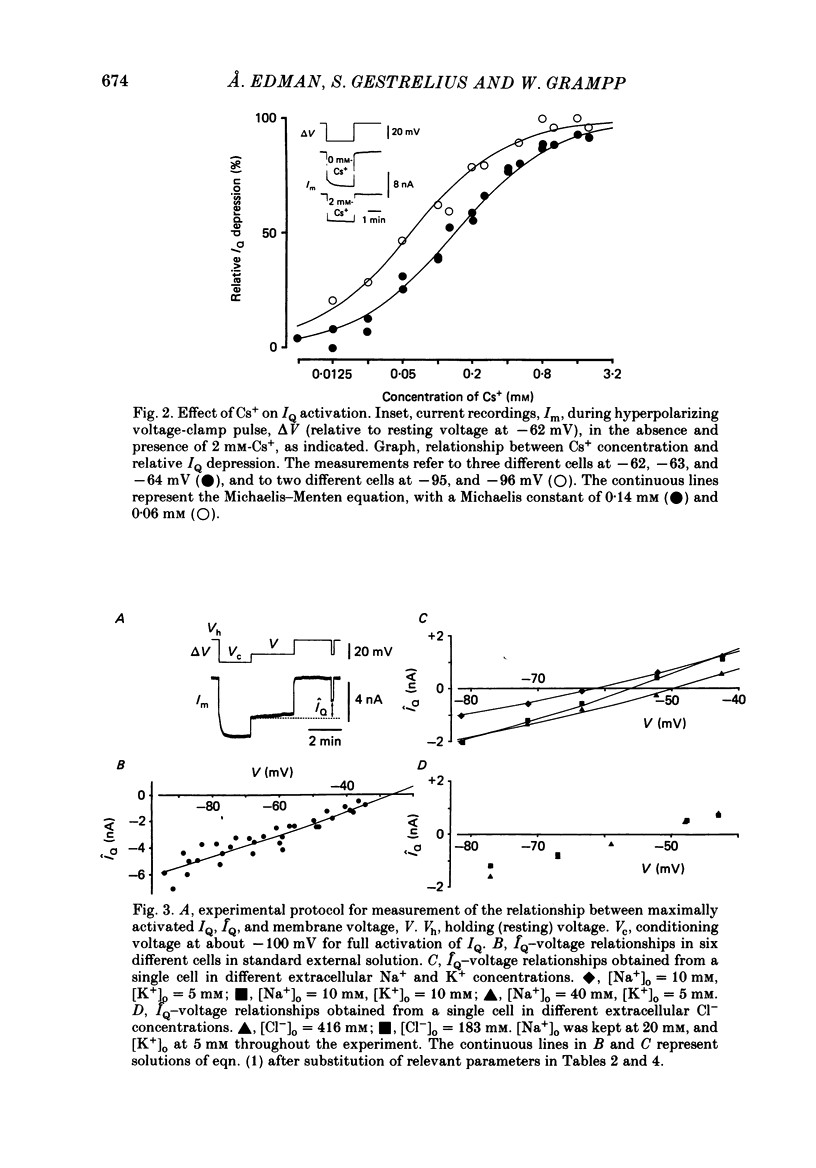
1. A polarization-induced membrane current, IQ, was investigated in the slowly adapting lobster stretch receptor neurone using conventional electrophysiological techniques including intracellular ion measurements. 2. The current was readily blocked by Cs+ in a voltage-dependent manner, but proved to be unaffected by tetrodotoxin, tetraethylammonium and 4-aminopyridine. 3. From an analysis of the ionic basis of IQ, it appeared that the current is carried by both Na+ and K+ through a membrane channel whose permeability for K+ is about six times larger than that for Na+ in a normal ionic environment. In the presence of reduced external Na+ concentration the Q-channel increases its permeability for both Na+ and K+, but more so for Na+ than for K+. 4. Kinetically, IQ was found to be characterized by a steep sigmoidal relationship between membrane voltage and steady-state current activation, and by a bell-shaped relationship between membrane voltage and the time constant of the exponential phase of current activation or deactivation. A significant feature of the latter relationship is a tendency to level off at finite time-constant values in both strongly hyperpolarizing and strongly depolarizing voltage regions. 5. From the experiments a mathematical IQ model was inferred. This model was based on constant-field and channel-gating kinetics involving a voltage-dependent reaction step in series with a voltage-independent reaction step. The model was found to successfully reproduce IQ behaviour in the living preparation. 6. Functionally, the activation of IQ was found to play a role in setting the cell's resting polarization and membrane excitability. This function was inferred from experiments on unimpaled cells in which it was possible to demonstrate some overlap between the voltage ranges of IQ activation and impulse initiation. In addition, in impaled cells the activation of IQ was found to cause some shortening of post-tetanic membrane hyperpolarization and to accelerate, thereby, the post-tetanic restoration of membrane excitability to control levels.
Full text
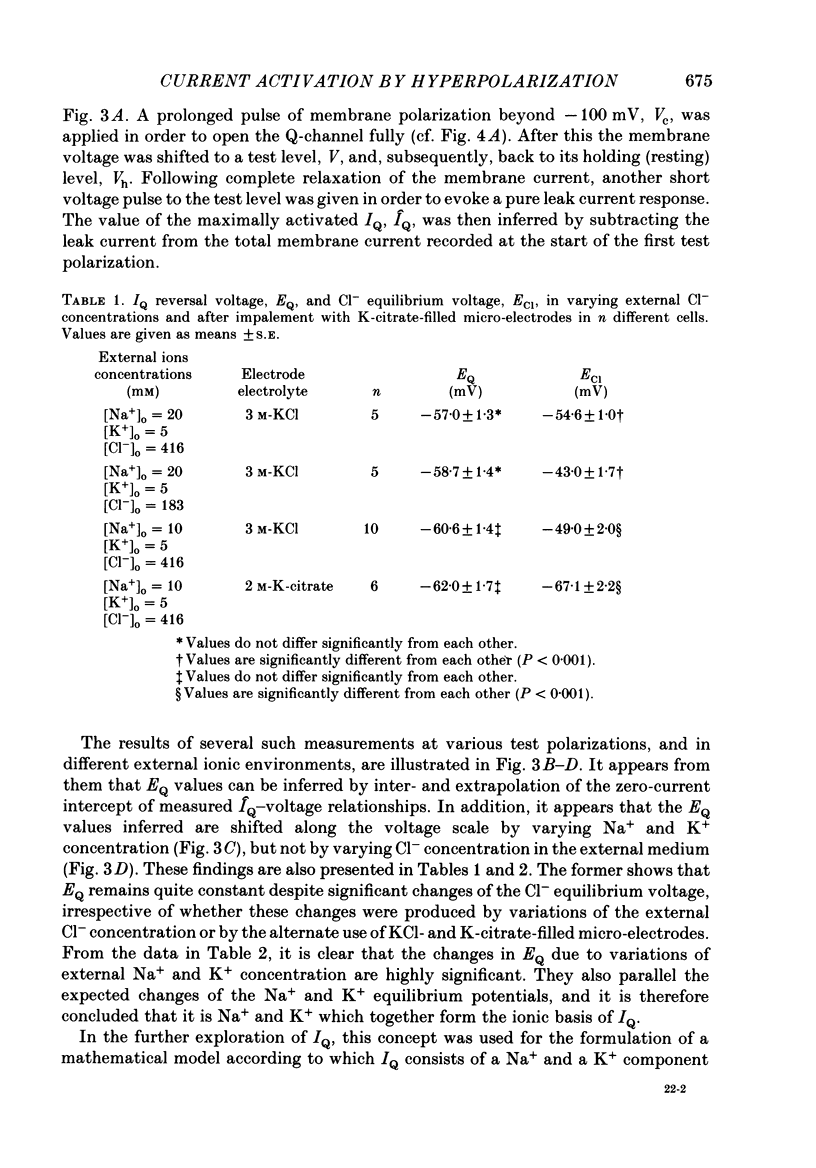
PDF


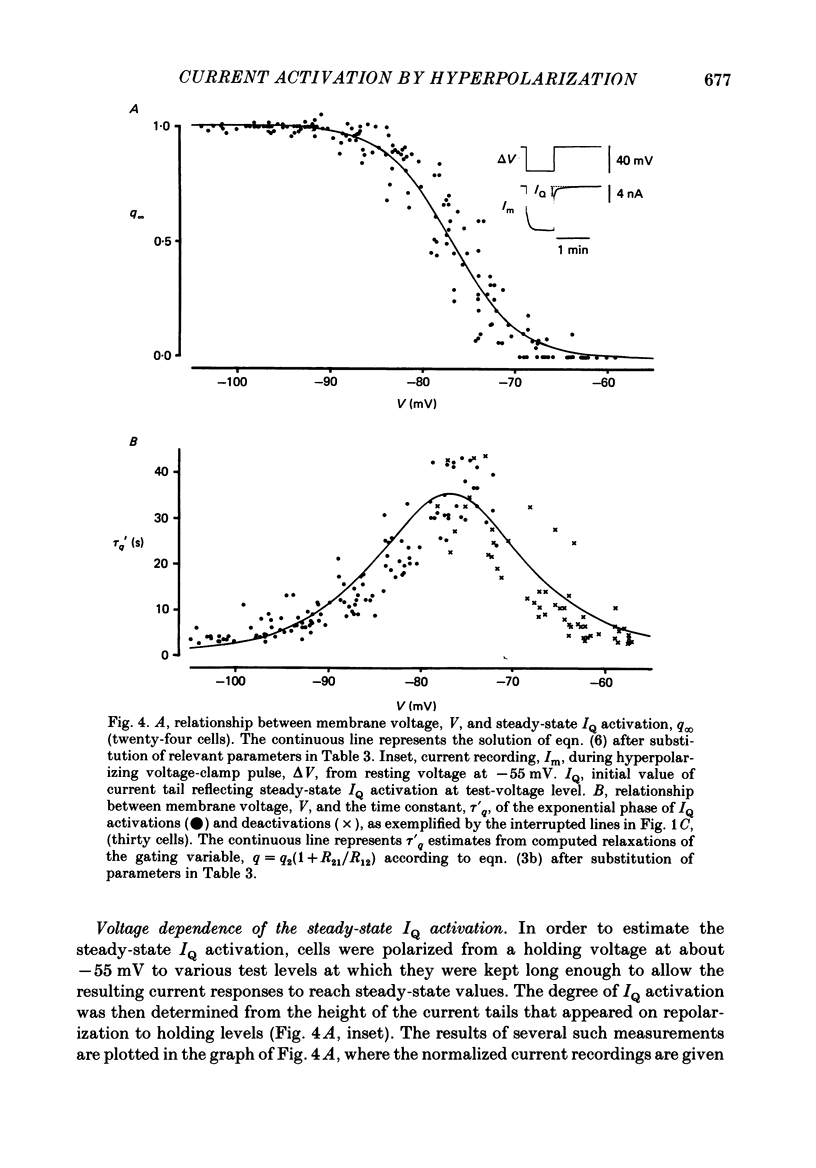

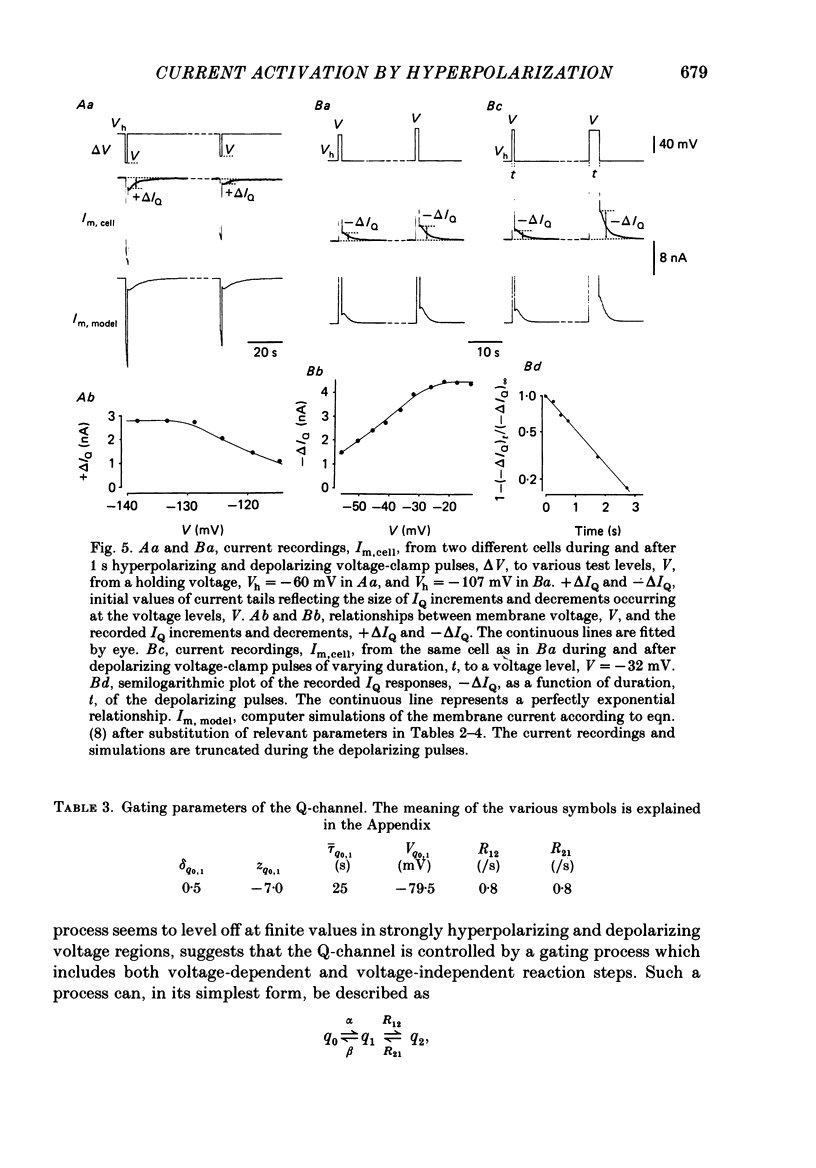

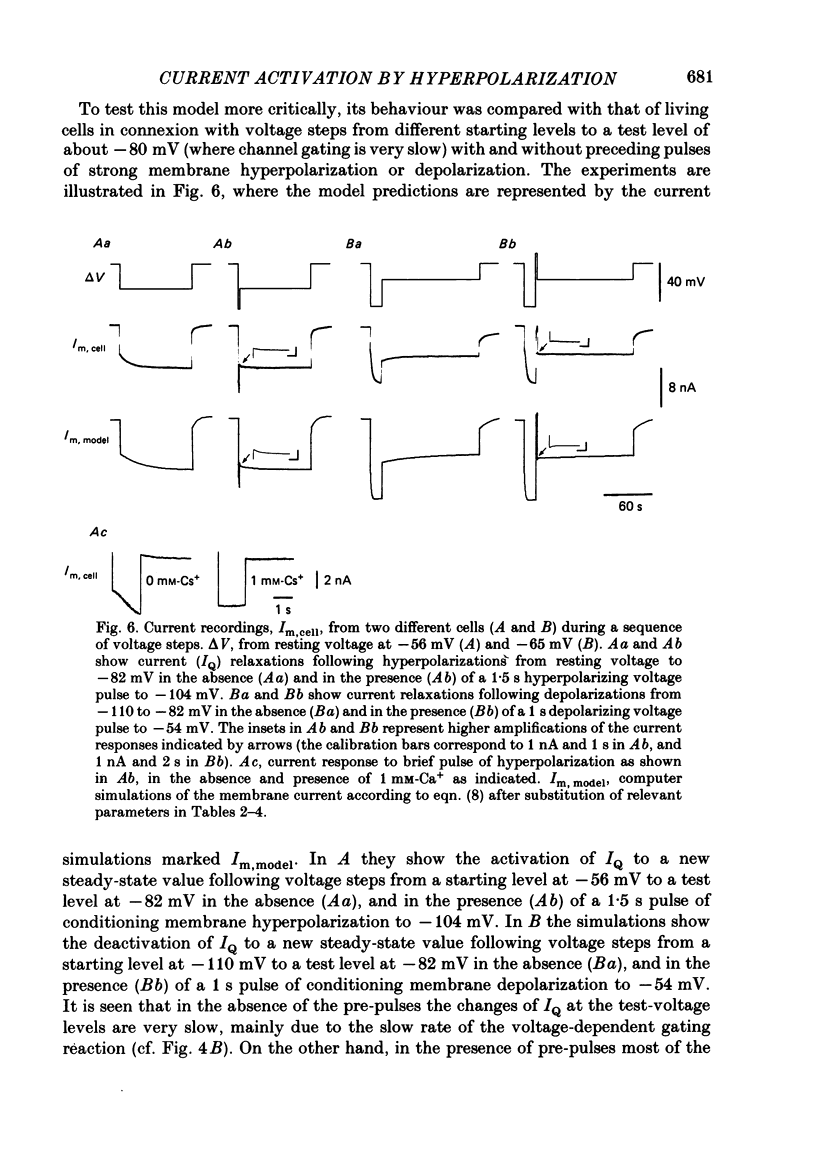

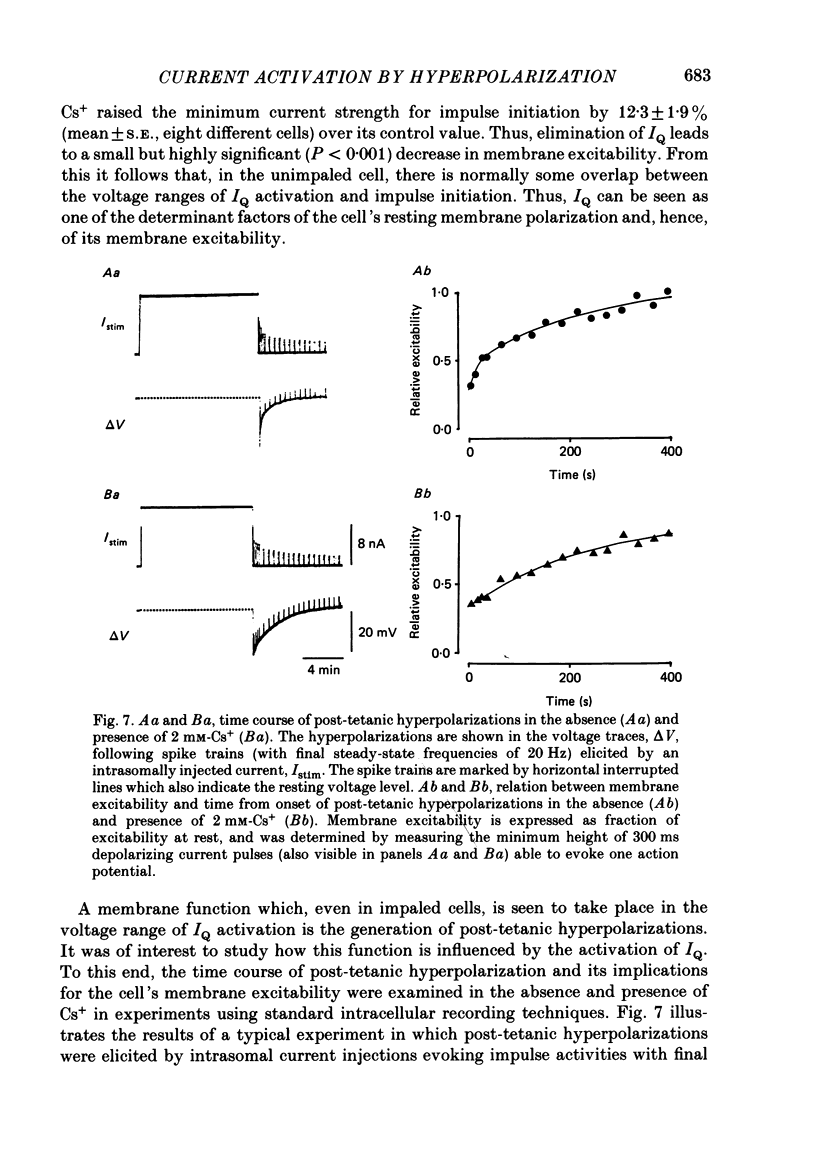







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Kimura J., Noble D., Noble S. J., Taupignon A. The ionic currents underlying pacemaker activity in rabbit sino-atrial node: experimental results and computer simulations. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):329–347. doi: 10.1098/rspb.1984.0067. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Characterization of a chloride conductance activated by hyperpolarization in Aplysia neurones. J Physiol. 1983 Sep;342:277–308. doi: 10.1113/jphysiol.1983.sp014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982 Aug;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of the pace-maker current kinetics in calf Purkinje fibres. J Physiol. 1984 Mar;348:341–367. doi: 10.1113/jphysiol.1984.sp015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earm Y. E., Shimoni Y., Spindler A. J. A pace-maker-like current in the sheep atrium and its modulation by catecholamines. J Physiol. 1983 Sep;342:569–590. doi: 10.1113/jphysiol.1983.sp014869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman A., Gestrelius S., Grampp W. Analysis of gated membrane currents and mechanisms of firing control in the rapidly adapting lobster stretch receptor neurone. J Physiol. 1987 Mar;384:649–669. doi: 10.1113/jphysiol.1987.sp016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman A., Gestrelius S., Grampp W. Transmembrane ion balance in slowly and rapidly adapting lobster stretch receptor neurones. J Physiol. 1986 Aug;377:171–191. doi: 10.1113/jphysiol.1986.sp016180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W. Impulse firing in the slowly adapting stretch receptor neurone of lobster and its numerical simulation. Acta Physiol Scand. 1983 Jul;118(3):253–261. doi: 10.1111/j.1748-1716.1983.tb07269.x. [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W. Kinetics of the TEA and 4-AP sensitive K+ current in the slowly adapting lobster stretch receptor neurone. Acta Physiol Scand. 1983 Jun;118(2):125–134. doi: 10.1111/j.1748-1716.1983.tb07252.x. [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W., Sjölin L. Subthreshold and near-threshold membrane currents in lobster stretch receptor neurones. J Physiol. 1981 Jan;310:191–203. doi: 10.1113/jphysiol.1981.sp013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hart G. The kinetics and temperature dependence of the pace-maker current if in sheep Purkinje fibres. J Physiol. 1983 Apr;337:401–416. doi: 10.1113/jphysiol.1983.sp014631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid Larsen E., Kristensen P. Properties of a conductive cellular chloride pathway in the skin of the toad (Bufo bufo). Acta Physiol Scand. 1978 Jan;102(1):1–21. doi: 10.1111/j.1748-1716.1978.tb06041.x. [DOI] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Morad M., Irisawa H. Does the "pacemaker current" generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 1983 May;397(3):190–194. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


