Abstract
1. Contractile, enzyme-histochemical and morphometrical properties of muscle fibres were studied in single motor units of tibialis anterior (t.a.) and soleus muscles in young (3-6 months) and old (20-24 months) male albino rats. The technique of measuring glycogen depletion as a marker of previous muscle contraction was used for direct correlation of enzyme-histochemical and contractile parameters within single motor units of the fast- and slow-twitch type. 2. In t.a., the fast-twitch motor units covered 18 +/- 9 and 22 +/- 16% (P = not significant, n.s.) of t.a. cross-sections, included 148 +/- 65 and 162 +/- 63 muscle fibres per unit (P = n.s.) and had a cross-sectional area of 0.50 +/- 0.32 and 0.44 +/- 0.22 mm2 (P = n.s.) in the young and old animals, respectively (means +/- S.D.). 3. In soleus, the slow-twitch motor units covered 53 +/- 11 and 71 +/- 12% (P = n.s.), included 55 +/- 10 and 83 +/- 13 muscle fibres per unit (P less than 0.01) and had a total cross-sectional area of 0.14 +/- 0.02 and 0.22 +/- 0.06 mm2 (P less than 0.01) in the young and the old animals, respectively. The calculated number of motor units in soleus accordingly decreased (P less than 0.01) from 49 +/- 10 in the young to 29 +/- 10 in the old animals resulting in a loss of muscle fibres and an increased innervation ratio in old age (mean +/- S.D.). 4. Clusters of more than three muscle fibres were rarely seen in any of the glycogen-depleted motor units in either the young or the old animals. However, in the slow-twitch motor units of old animals the muscle fibres were less randomly distributed within the motor unit territory (P less than 0.05), indicating a denervation-reinnervation process. 5. The contraction and half-relaxation times of the isometric twitch were significantly prolonged in old age. In 274 randomly isolated single motor units of t.a. the contraction time increased from 13 +/- 1 in young animals to 17 +/- 3 ms in old ones and the half-relaxation time from 12 +/- 2 to 16 +/- 5 ms (P less than 0.01 in both cases). In 236 randomly isolated soleus single motor units, the contraction and half-relaxation times increased (P less than 0.001) from 24 +/- 5 to 31 +/- 7 ms and from 26 +/- 8 to 35 +/- 9 ms, respectively (mean +/- S.D.).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















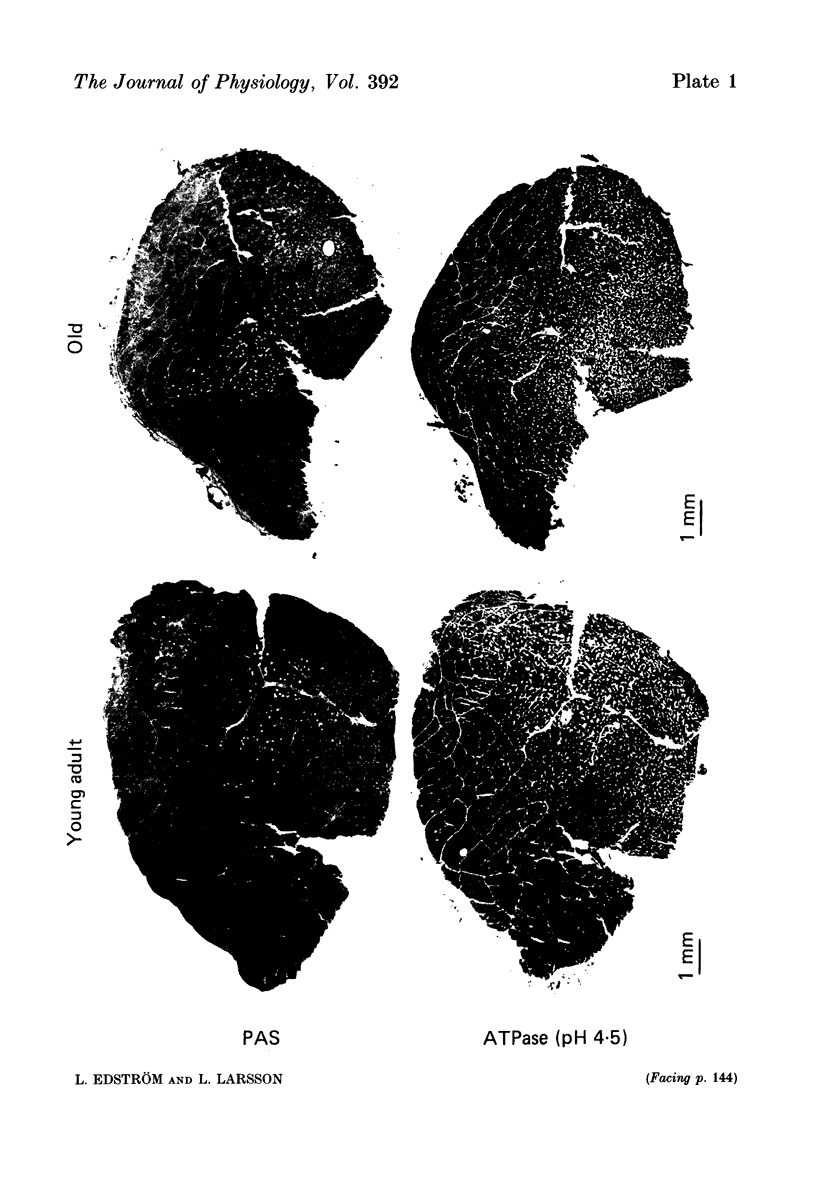


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker B. Q., Kelly S. S., Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983 Jun;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger A. Y., McComas A. J., Elder G. B. Physiological properties of two antagonist human muscle groups. Eur J Appl Physiol Occup Physiol. 1983;51(3):381–393. doi: 10.1007/BF00429075. [DOI] [PubMed] [Google Scholar]
- Borg J. Properties of single motor units of the extensor digitorum brevis in elderly humans. Muscle Nerve. 1981 Sep-Oct;4(5):429–434. doi: 10.1002/mus.880040513. [DOI] [PubMed] [Google Scholar]
- Brody I. A. Regulation of isometric contraction in skeletal muscle. Exp Neurol. 1976 Mar;50(3):673–683. doi: 10.1016/0014-4886(76)90036-4. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969 Jun;17(6):431–432. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979 May-Jun;2(3):202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., McComas A. J., Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973 Apr;36(2):174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. T., White M. J., Young K. Electrically evoked and voluntary maximal isometric tension in relation to dynamic muscle performance in elderly male subjects, aged 69 years. Eur J Appl Physiol Occup Physiol. 1983;51(1):37–43. doi: 10.1007/BF00952535. [DOI] [PubMed] [Google Scholar]
- De Coster W., De Reuck J., Sieben G., Vander Eecken H. Early ultrastructural changes in aging rat gastrocnemius muscle: a stereologic study. Muscle Nerve. 1981 Mar-Apr;4(2):111–116. doi: 10.1002/mus.880040206. [DOI] [PubMed] [Google Scholar]
- Dulhunty A., Valois A. Indentations in the terminal cisternae of amphibian and mammalian skeletal muscle fibers. J Ultrastruct Res. 1983 Jul;84(1):34–49. doi: 10.1016/s0022-5320(83)90084-9. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V. Motor unit in old age. Nature. 1966 Feb 26;209(5026):921–922. doi: 10.1038/209921b0. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V., Vysokocil F. Age changes in cross striated muscle of the rat. J Physiol. 1971 Jul;216(2):331–343. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriman D. G., Taverner D., Woolf A. L. Ekbom's syndrome and burning paraesthesiae. A biopsy study by vital staining and electron microscopy of the intramuscular innervation with a note on age changes in motor nerve endings in distal muscles. Brain. 1970;93(2):393–406. doi: 10.1093/brain/93.2.393. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Freiman D. G. An improved method of fixation for formalin-sensitive enzymes with special reference to myosin adenosine triphosphatase. J Histochem Cytochem. 1966 Aug;14(8):577–581. doi: 10.1177/14.8.577. [DOI] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L., Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E., Thornell L. E. Contraction time, histochemical type, and terminal cisternae volume of rat motor units. Muscle Nerve. 1983 Feb;6(2):149–153. doi: 10.1002/mus.880060211. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci. 1986 Nov;76(1):69–89. doi: 10.1016/0022-510x(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Larsson L. Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol Scand Suppl. 1978;457:1–36. [PubMed] [Google Scholar]
- McComas A. J., Sica R. E., Campbell M. J. "Sick" motoneurones. A unifying concept of muscle disease. Lancet. 1971 Feb 13;1(7694):321–326. doi: 10.1016/s0140-6736(71)91045-2. [DOI] [PubMed] [Google Scholar]
- Nelson R. M., Soderberg G. L., Urbscheit N. L. Alteration of motor-unit discharge characteristics in aged humans. Phys Ther. 1984 Jan;64(1):29–34. doi: 10.1093/ptj/64.1.29. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Serratrice G., Roux H., Aquaron R. Proximal muscular weakness in elderly subjects. Report of 12 cases. J Neurol Sci. 1968 Sep-Oct;7(2):275–299. doi: 10.1016/0022-510x(68)90149-4. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Walton J. N., Rebeiz J. J. The effects of ageing and of cachexia upon skeletal muscle. A histopathological study. J Neurol Sci. 1969 Sep-Oct;9(2):321–346. doi: 10.1016/0022-510x(69)90079-3. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. Histochemical and ultrastructural changes in senile human skeletal muscle. J Am Geriatr Soc. 1977 Mar;25(3):125–131. doi: 10.1111/j.1532-5415.1977.tb00274.x. [DOI] [PubMed] [Google Scholar]




