Abstract
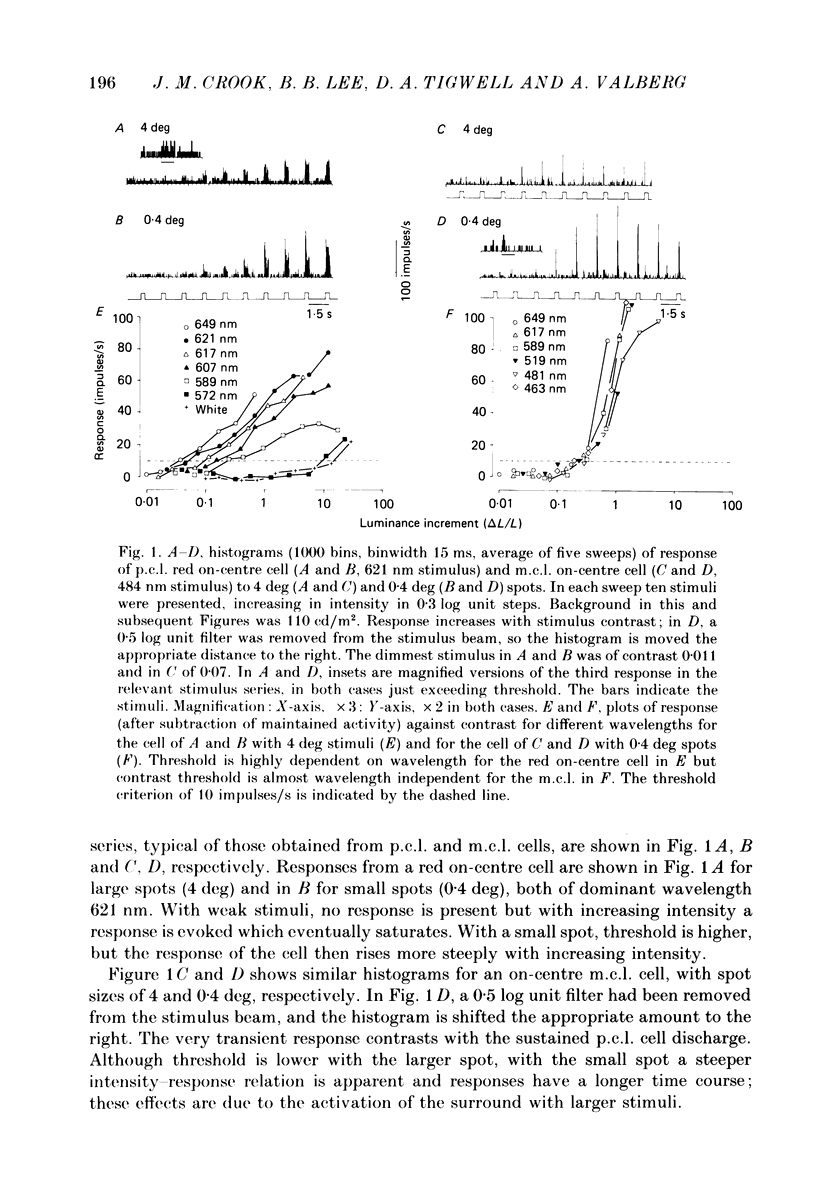
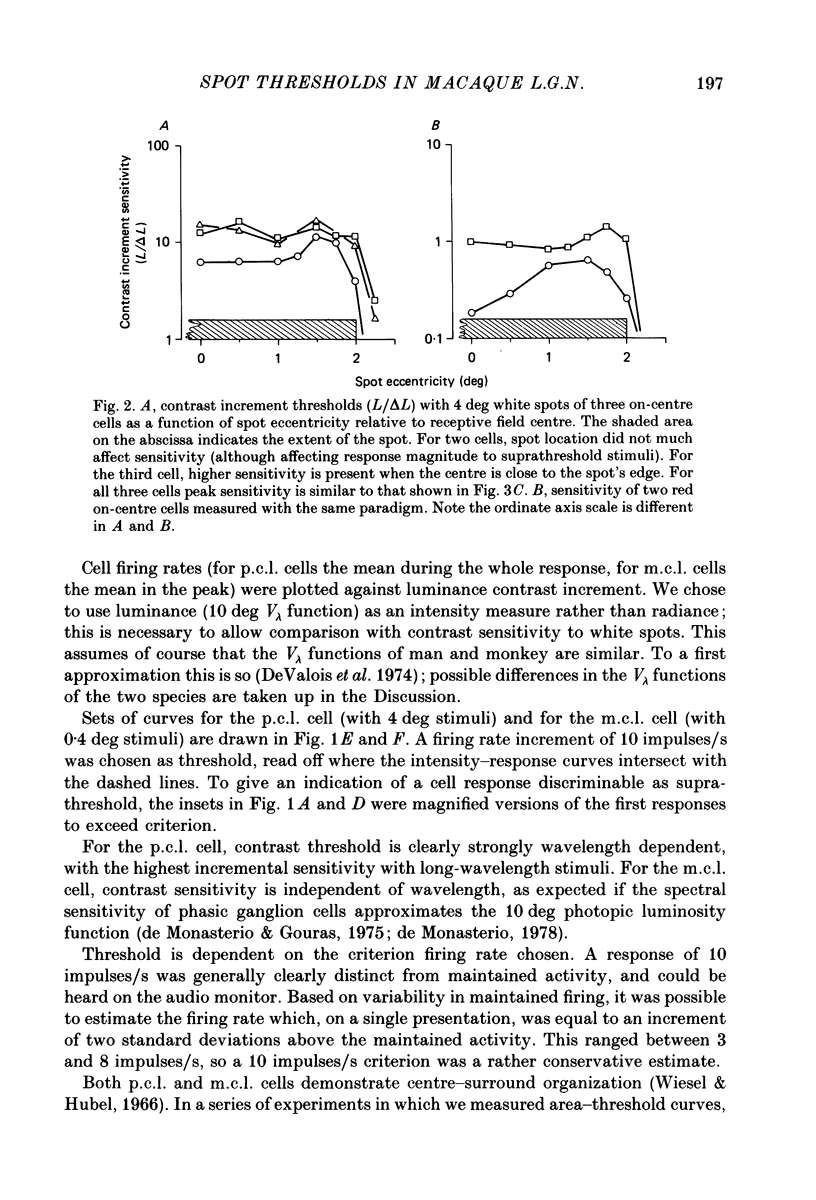
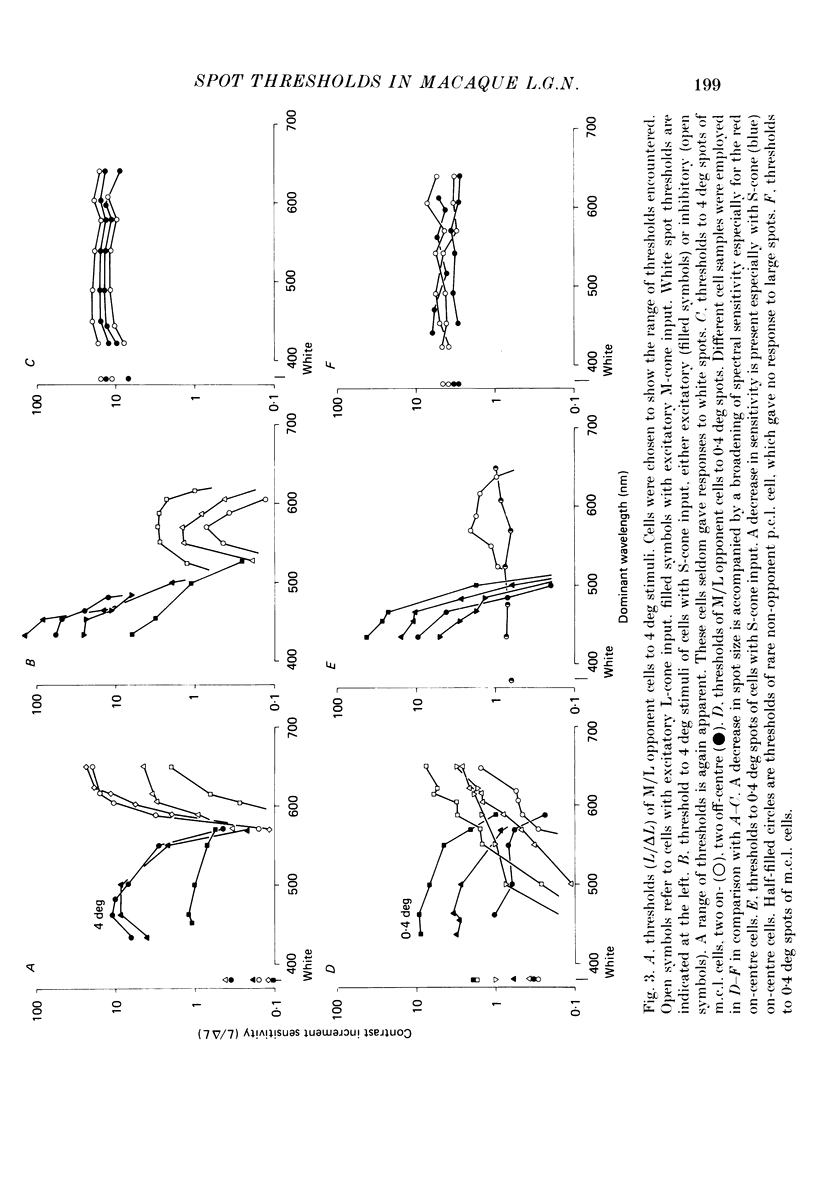
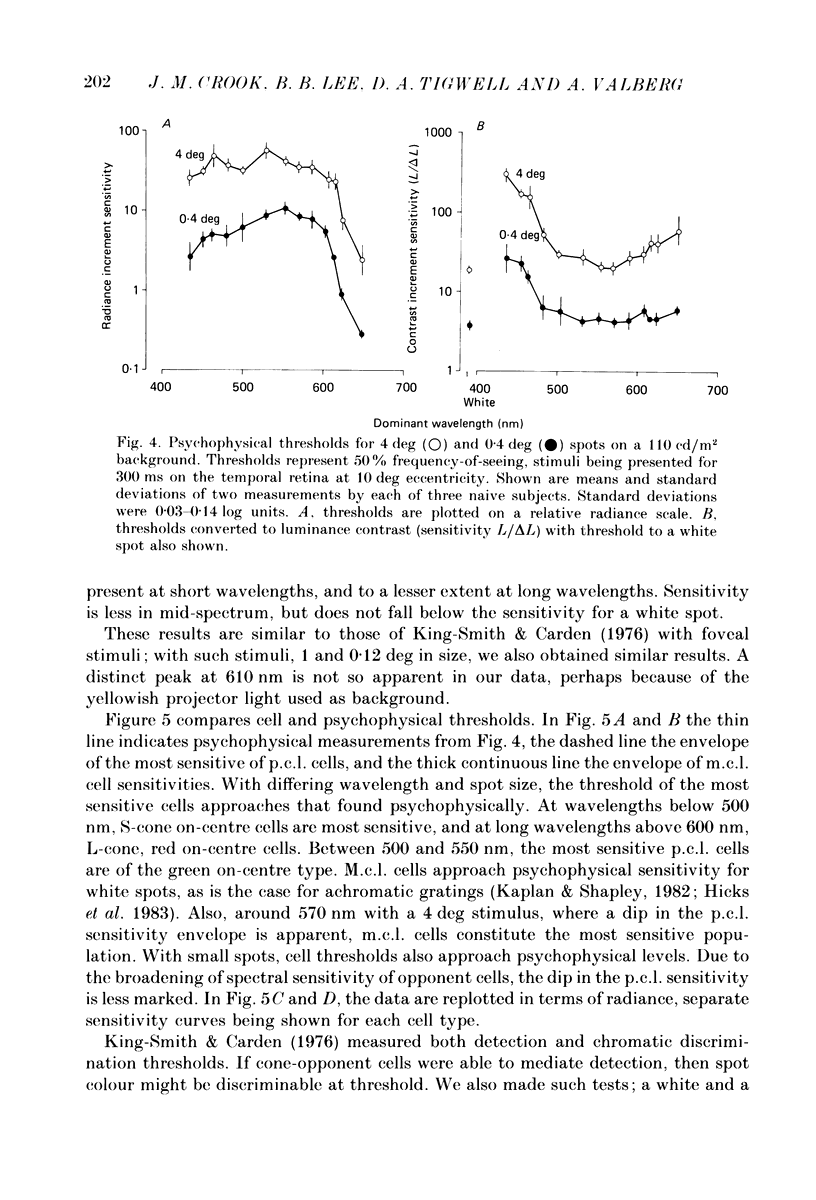
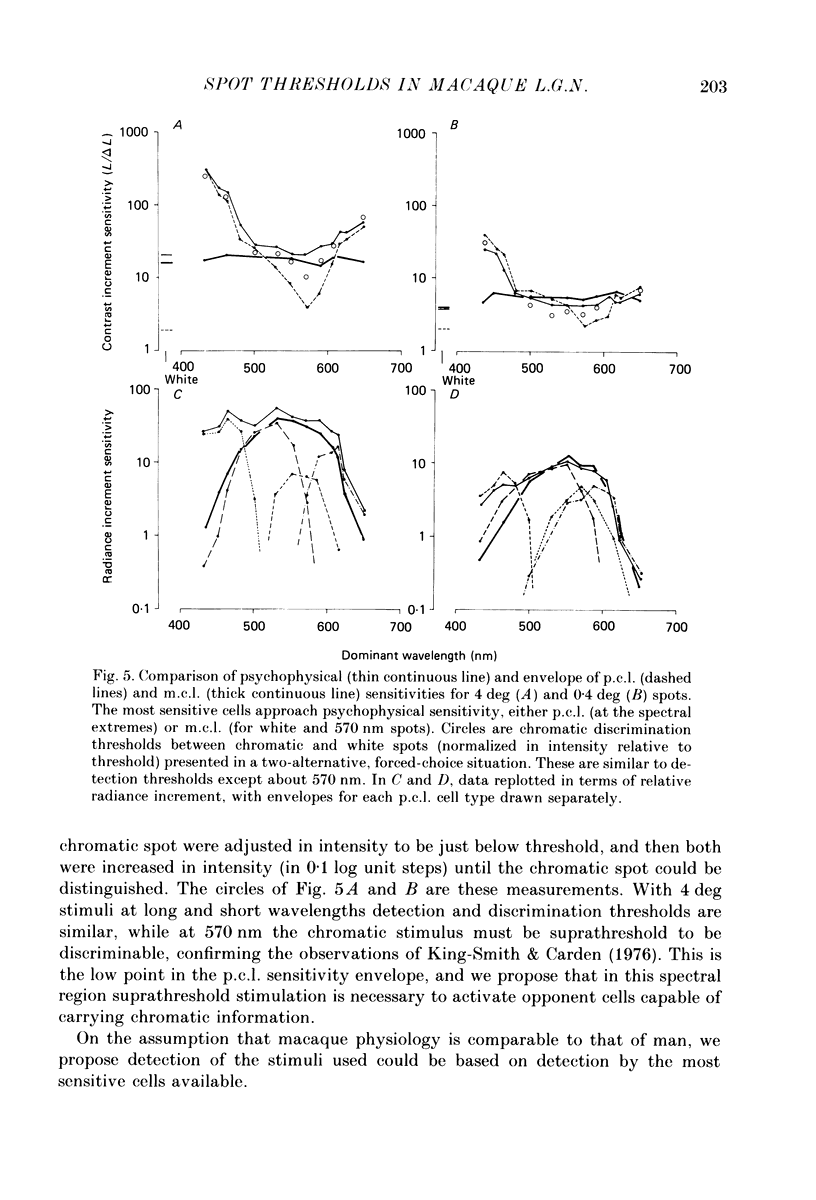
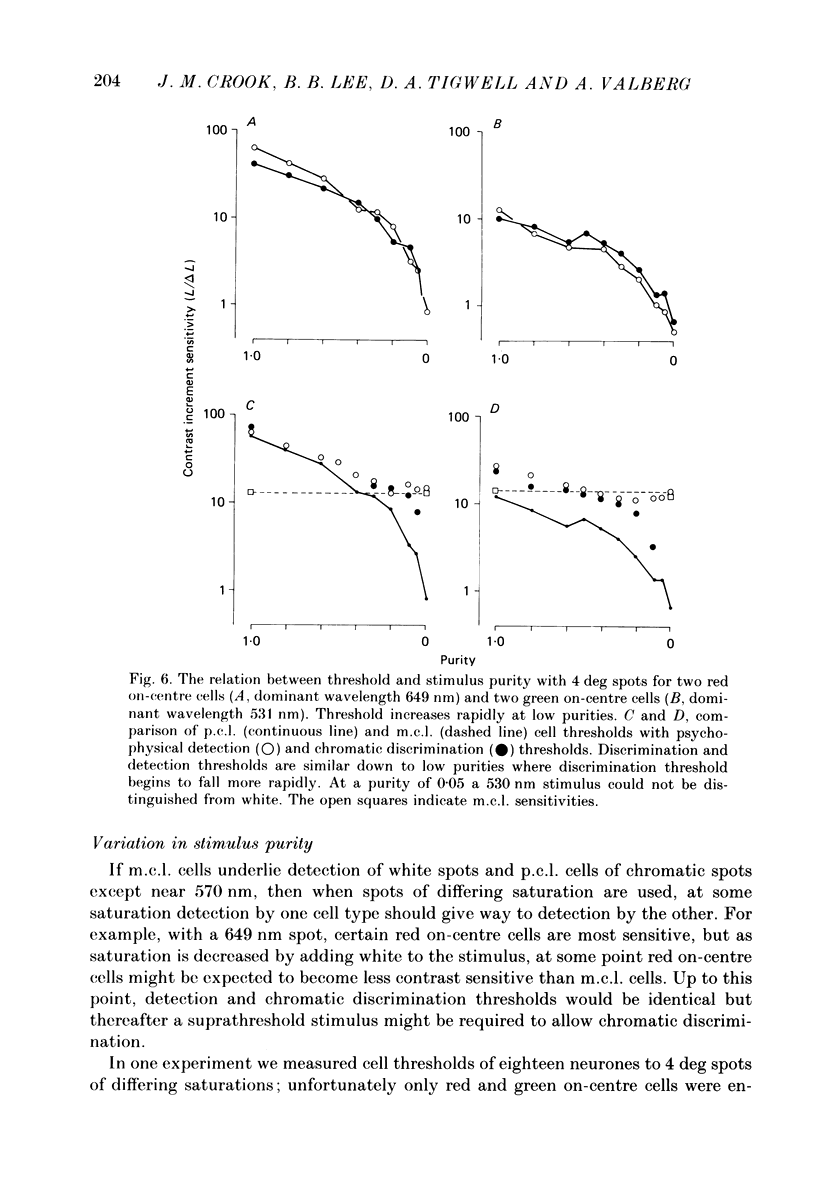
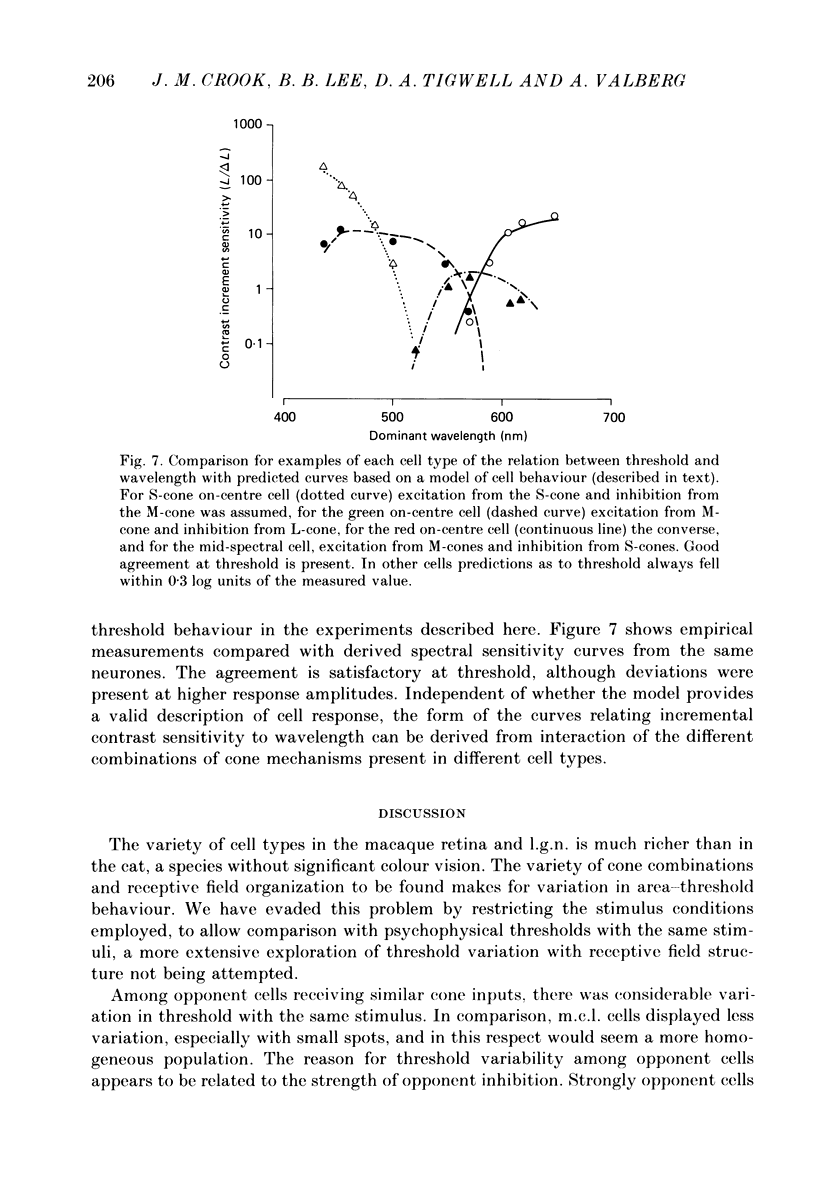
1. The relation between wavelength and psychophysical threshold for chromatic spots on a white background provides evidence for the existence of chromatic channels in the primate visual system. To find the physiological substrate of this task, we compared increment thresholds of different cell types in the macaque lateral geniculate nucleus with human psychophysical thresholds to the same stimuli, using two spot sizes, 4 and 0.4 deg. 2. At different wavelengths, different opponent cell classes in the parvocellular layers of the nucleus were most sensitive, so that at long wavelengths (greater than 600 nm) red on-centre cells were most sensitive, while at short wavelengths (less than 500 nm) S-cone, blue on-centre cells were most sensitive, from 500 to about 550 nm green on-centre cells being most sensitive. A rare cell type with inhibition from S-cones was most sensitive at about 570 nm, although its maximum contrast increment sensitivity was poor compared with that of other cell types. Variation in strength of cone opponency caused a considerable range in threshold in each of the opponent cell classes of the parvocellular layers. 3. On- and off-centre cells from the magnocellular layers were more sensitive than opponent cells to white and yellow spots (as is the case with achromatic gratings). 4. With different wavelengths and spot sizes, the most sensitive cells found approached (to within 0.1-0.3 log units) human psychophysical sensitivity, suggesting that the most sensitive cells available may underlie detection. 5. Measurements of psychophysical chromatic discrimination thresholds, both with nearly monochromatic spots and with spots of differing saturation (purity), support this hypothesis. When magnocellular cell sensitivity corresponded to psychophysical threshold, a suprathreshold stimulus, capable of activating opponent cells, was required for chromatic discrimination.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowmaker J. K., Dartnall H. J., Mollon J. D. Microspectrophotometric demonstration of four classes of photoreceptor in an old world primate, Macaca fascicularis. J Physiol. 1980 Jan;298:131–143. doi: 10.1113/jphysiol.1980.sp013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J. Visual pigments of rods and cones in a human retina. J Physiol. 1980 Jan;298:501–511. doi: 10.1113/jphysiol.1980.sp013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Lee B. B., Elepfandt A. A quantitative study of chromatic organisation and receptive fields of cells in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1979 May 2;35(3):527–545. doi: 10.1007/BF00236770. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H. C., Polson M. C., Mead W. R., Hull E. M. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974 Jan;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Krauskopf J., Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M. A., Hood D. C. Detection and discrimination of small, brief lights: variable tuning of opponent channels. Vision Res. 1984;24(3):175–181. doi: 10.1016/0042-6989(84)90119-6. [DOI] [PubMed] [Google Scholar]
- Graham N. Visual detection of aperiodic spatial stimuli by probability summation among narrowband channels. Vision Res. 1977;17(5):637–652. doi: 10.1016/0042-6989(77)90140-7. [DOI] [PubMed] [Google Scholar]
- HURVICH L. M., JAMESON D. An opponent-process theory of color vision. Psychol Rev. 1957 Nov;64, Part 1(6):384–404. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Lee B. B., Vidyasagar T. R. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983 Apr;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith P. E., Carden D. Luminance and opponent-color contributions to visual detection and adaptation and to temporal and spatial integration. J Opt Soc Am. 1976 Jul;66(7):709–717. doi: 10.1364/josa.66.000709. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Virsu V., Creutzfeldt O. D. Linear signal transmission from prepotentials to cells in the macaque lateral geniculate nucleus. Exp Brain Res. 1983;52(1):50–56. doi: 10.1007/BF00237148. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Virsu V., Elepfandt A. Cell responses in dorsal layers of macaque lateral geniculate nucleus as a function of intensity and wavelength. J Neurophysiol. 1983 Oct;50(4):849–863. doi: 10.1152/jn.1983.50.4.849. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn B. J., Schnapf J. L., Baylor D. A. Spectral sensitivity of single cones in the retina of Macaca fascicularis. Nature. 1984 May 17;309(5965):264–266. doi: 10.1038/309264a0. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Sperling H. G., Crawford M. L., Espinoza S. Threshold spectral sensitivity of single neurons in the lateral geniculate nucleus and of performing monkeys. Mod Probl Ophthalmol. 1978;19:2–18. [PubMed] [Google Scholar]
- Sperling H. G., Harwerth R. S. Red-green cone interactions in the increment-threshold spectral sensitivity of primates. Science. 1971 Apr 9;172(3979):180–184. doi: 10.1126/science.172.3979.180. [DOI] [PubMed] [Google Scholar]
- Teller D. Y. Linking propositions. Vision Res. 1984;24(10):1233–1246. doi: 10.1016/0042-6989(84)90178-0. [DOI] [PubMed] [Google Scholar]
- Valberg A., Lee B. B., Tigwell D. A. Neurones with strong inhibitory S-cone inputs in the macaque lateral geniculate nucleus. Vision Res. 1986;26(7):1061–1064. doi: 10.1016/0042-6989(86)90040-4. [DOI] [PubMed] [Google Scholar]
- Valberg A., Seim T., Lee B. B., Tryti J. Reconstruction of equidistant color space from responses of visual neurones of macaques. J Opt Soc Am A. 1986 Oct;3(10):1726–1734. doi: 10.1364/josaa.3.001726. [DOI] [PubMed] [Google Scholar]
- Virsu V., Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Exp Brain Res. 1979;37(3):475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]


