Abstract
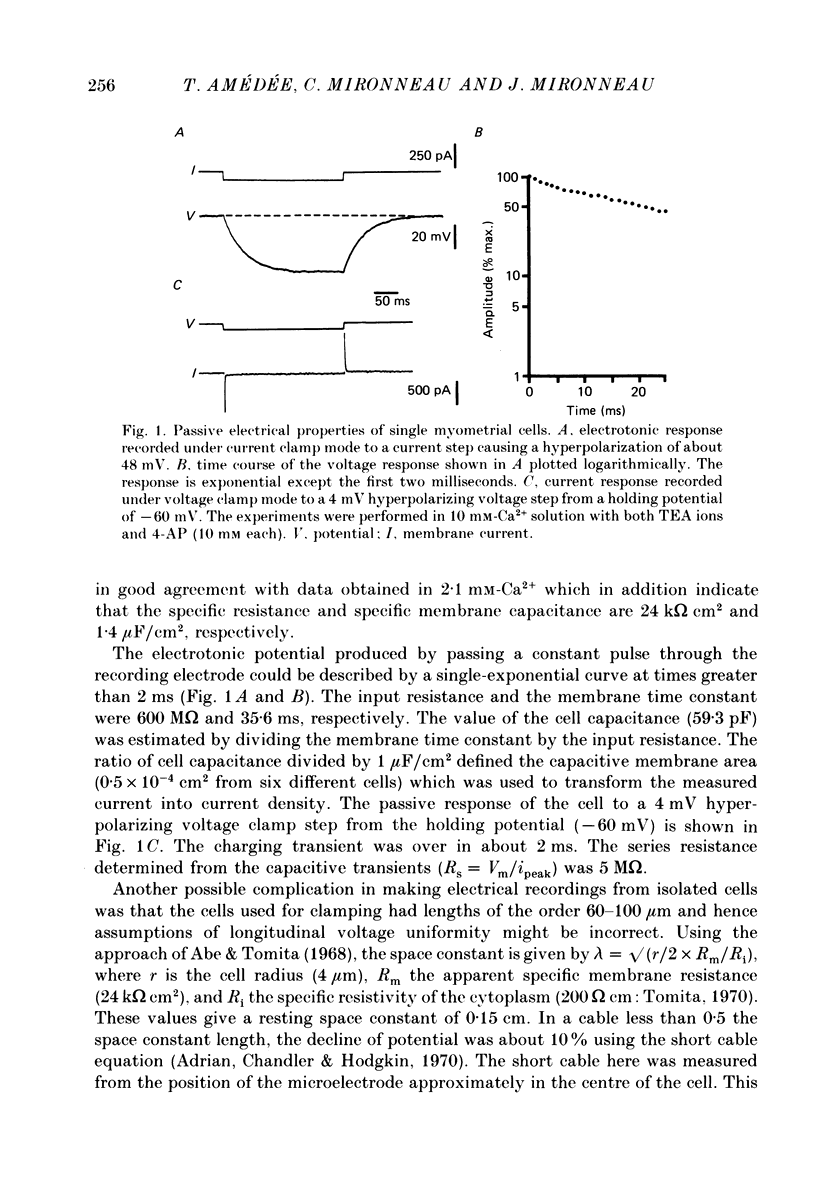
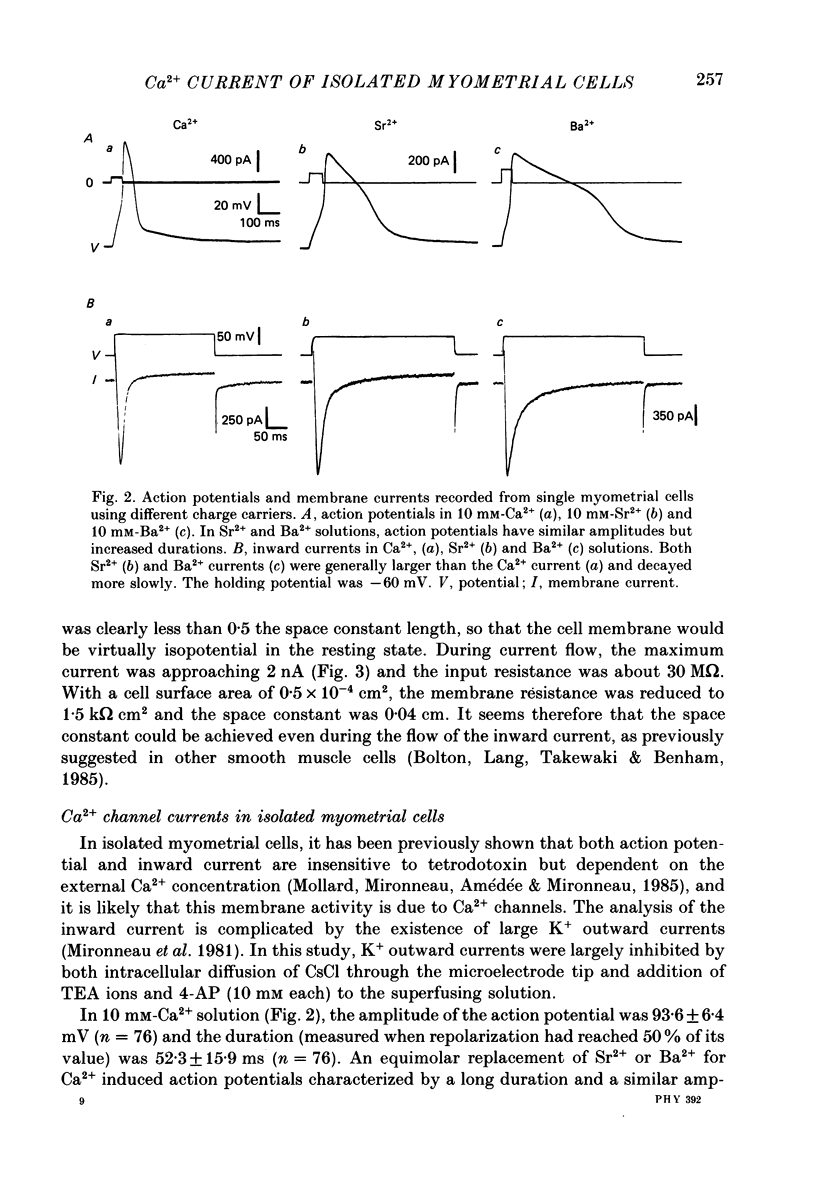
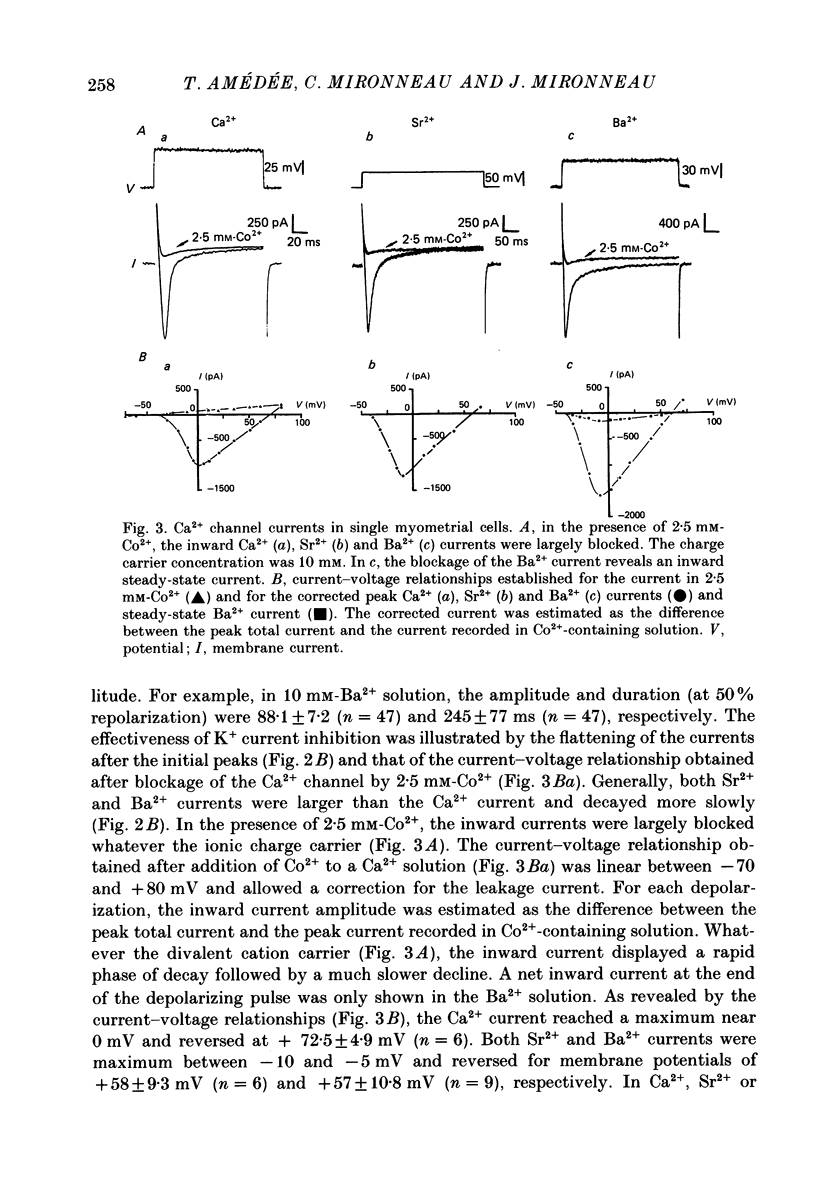
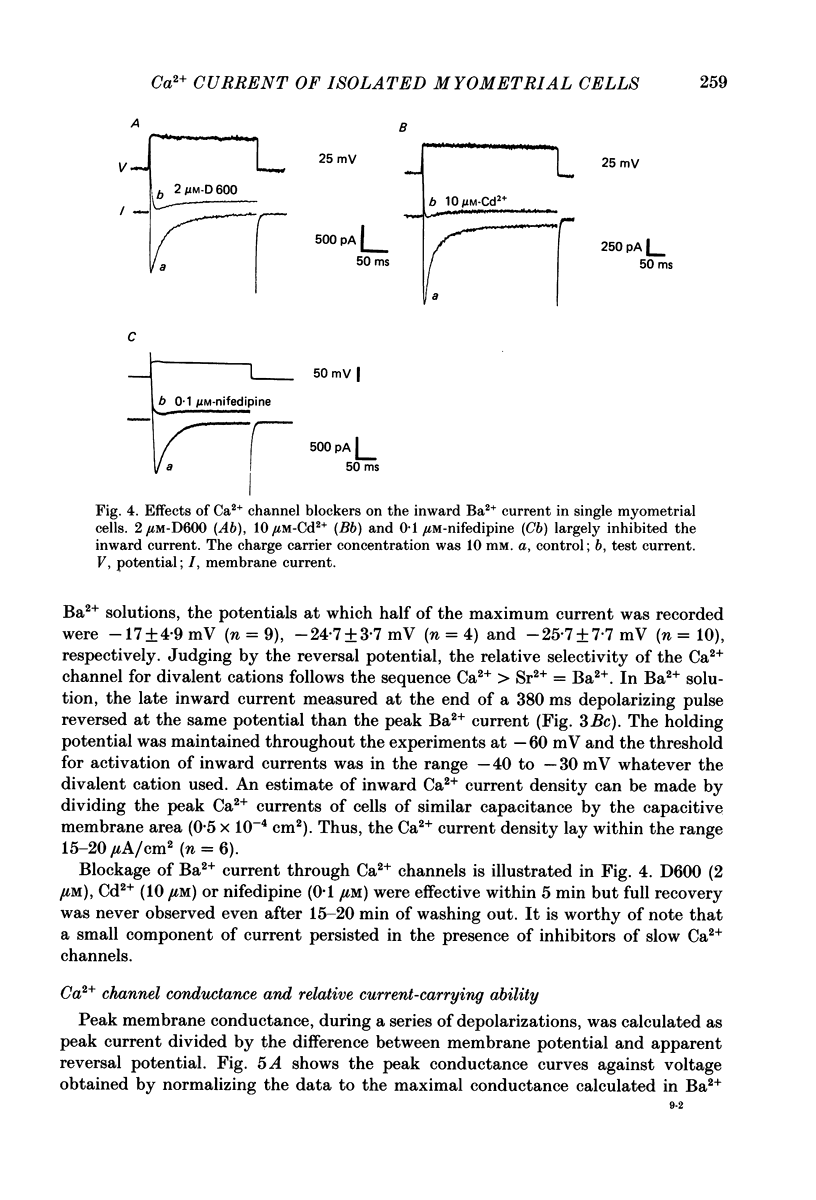
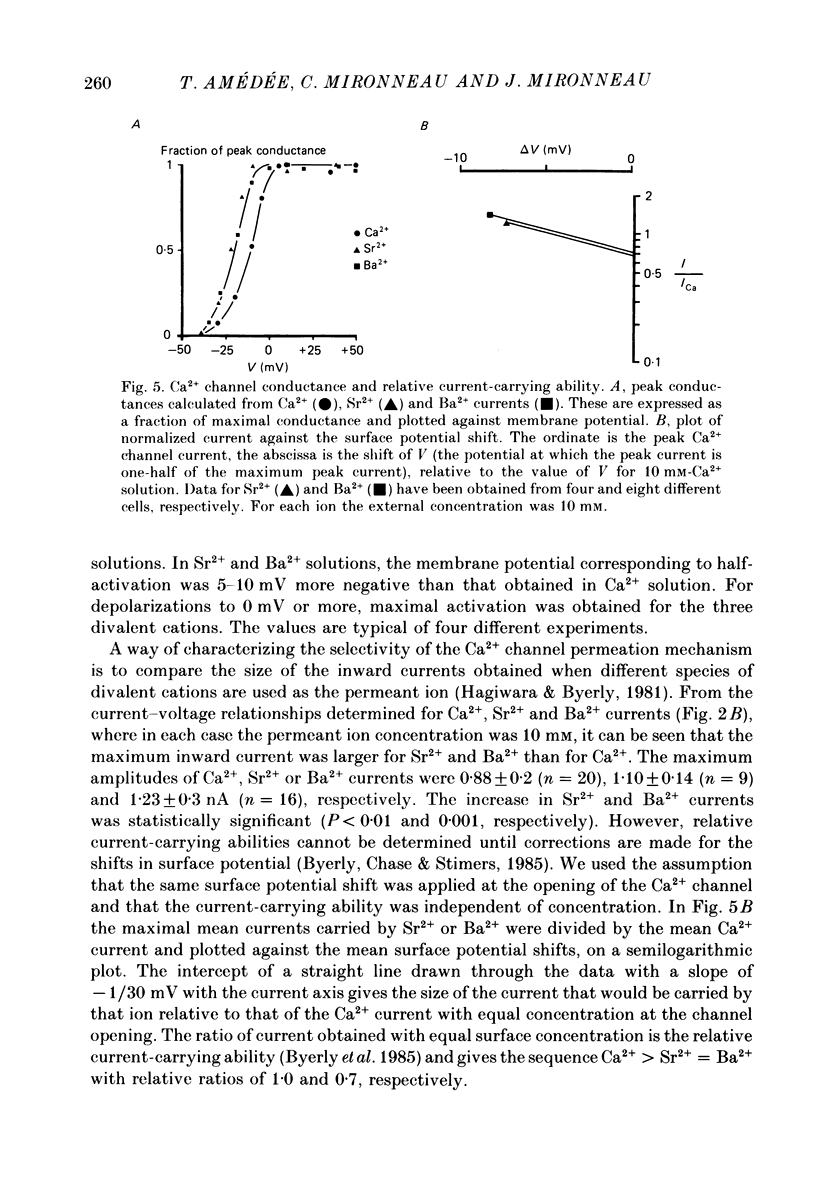
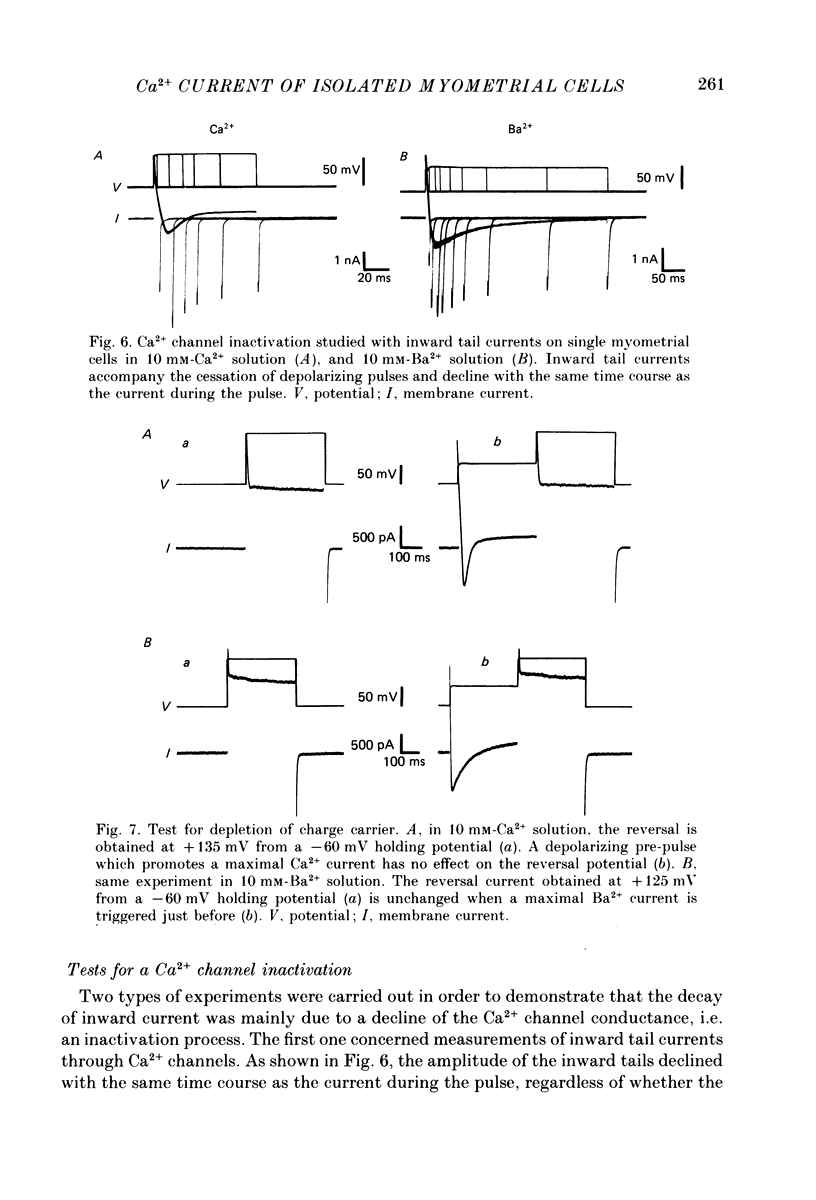
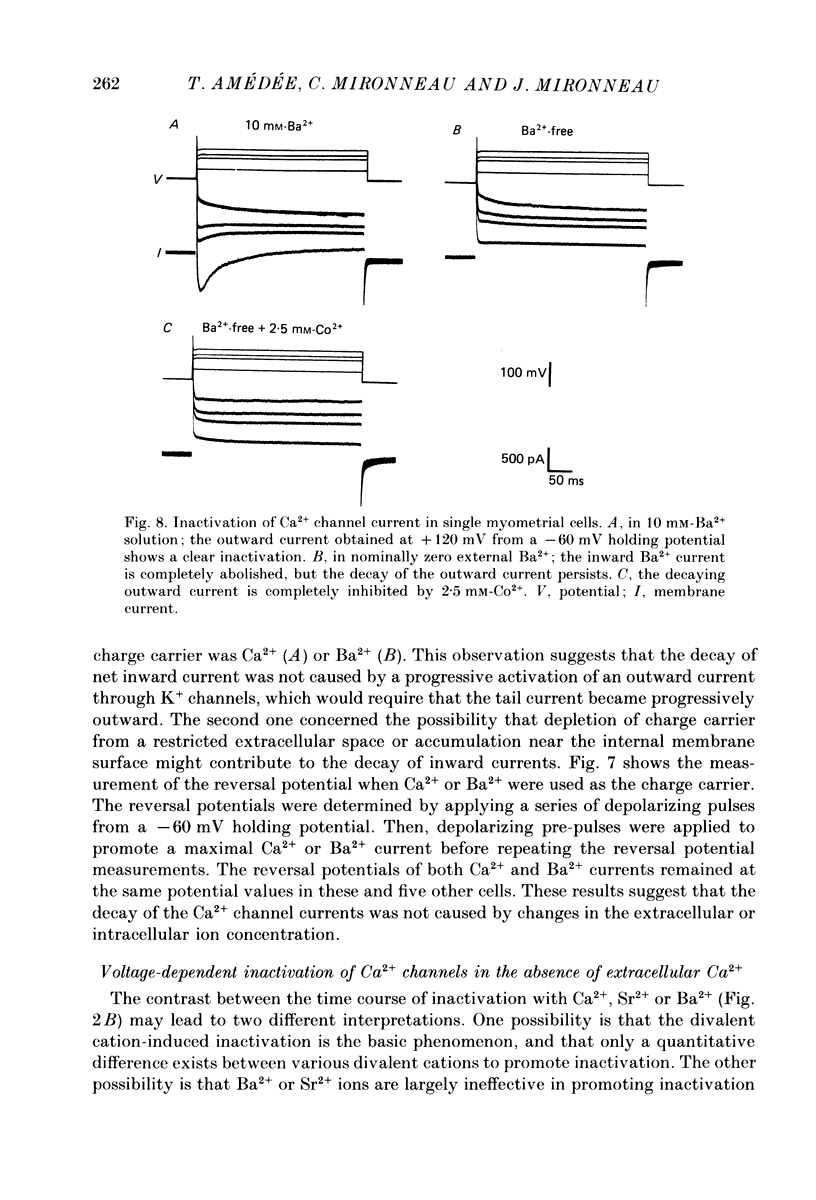
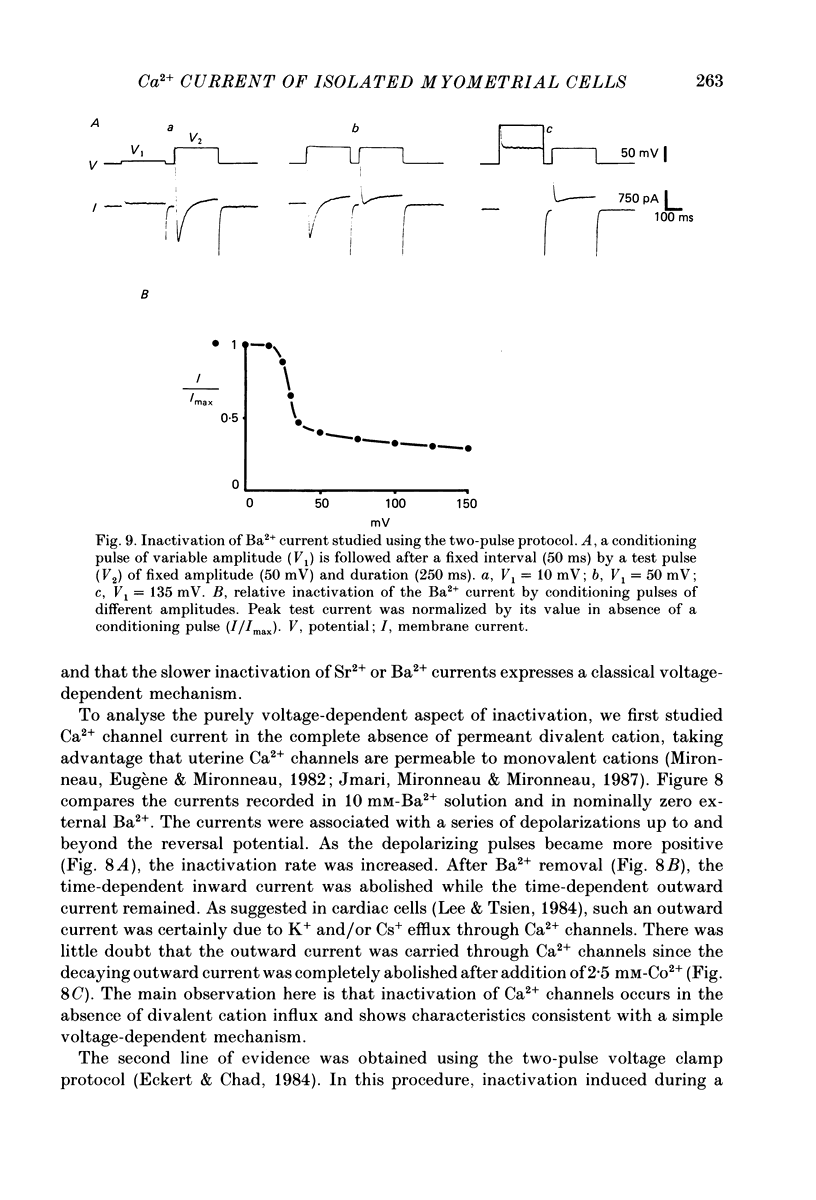
1. The passive and active electrical properties of pregnant rat single myometrial cells in short-term primary culture were analysed using a single-electrode voltage or current clamp. 2. Action potentials and membrane currents were recorded in the presence of tetraethylammonium chloride and 4-aminopyridine (10 mM each) and with Cs+ solution (4 M) in the microelectrode. 3. The voltage dependence, the action of Ca2+ antagonists and the effects of Sr2+ or Ba2+ substitution were studied. The peak Ca2+ current density was in the range 15-20 microA/cm2 in 10 mM-Ca2+ solution. 4. According to both measurement of the reversal potential of Ca2+ channel currents and comparison of the inward currents after correction for changing surface charge, the relative selectivity sequence of the Ca2+ channel for divalent cations was Ca2+ greater than Sr2+ = Ba2+. 5. The decay of Ca2+ channel current during a maintained depolarization was slowed when external Ca2+ was replaced by Sr2+ or Ba2+. The decay reflected an inactivation of Ca2+ channel conductance, as assessed by the decreased amplitude of inward tail currents following progressively longer depolarizations and the stable value of the reversal potential when Ca2+ channel current was increased during conditioning pulses. 6. Voltage-dependent inactivation was illustrated by inactivation of outward Ca2+ channel current due to K+ and/or Cs+ efflux with external Ba2+ or in the absence of any permeant divalent cation. 7. The relationship between inactivation and the intracellular Ca2+ concentration was assessed by a double-pulse method. Conditioning pulses that produced maximal Ca2+ current induced maximal inactivation; with stronger depolarizations, inactivation decreased but was not completely prevented at the expected Ca2+ reversal potential. Increasing the amount of Ca2+ entering the cell during the pre-pulse reduced both amplitude and kinetics of test Ca2+ currents. These results were not observed with Ba2+ as the charge carrier. 8. Ca2+ channel current inactivation was best fitted by a two-exponential function. The fast time constant of inactivation was larger in Ba2+ solution than in Ca2+ solution but both time constants showed little variation with membrane potential. The slow time constants of inactivation were steeply voltage dependent.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Mironneau C., Mironneau J. Isolation and contractile responses of single pregnant rat myometrial cells in short-term primary culture and the effects of pharmacological and electrical stimuli. Br J Pharmacol. 1986 Aug;88(4):873–880. doi: 10.1111/j.1476-5381.1986.tb16261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Chase P. B., Stimers J. R. Permeation and interaction of divalent cations in calcium channels of snail neurons. J Gen Physiol. 1985 Apr;85(4):491–518. doi: 10.1085/jgp.85.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognard C., Lazdunski M., Romey G. Different types of Ca2+ channels in mammalian skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1986 Jan;83(2):517–521. doi: 10.1073/pnas.83.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. E., Suarez-Kurtz G., Kaczorowski G. J., Katz G. M., Reuben J. P. Two calcium currents in a smooth muscle cell line. Am J Physiol. 1986 Apr;250(4 Pt 2):H699–H703. doi: 10.1152/ajpheart.1986.250.4.H699. [DOI] [PubMed] [Google Scholar]
- Fromm M., Schultz S. G. Some properties of KCl-filled microelectrodes: correlation of potassium "leakage" with tip resistance. J Membr Biol. 1981;62(3):239–244. doi: 10.1007/BF01998169. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
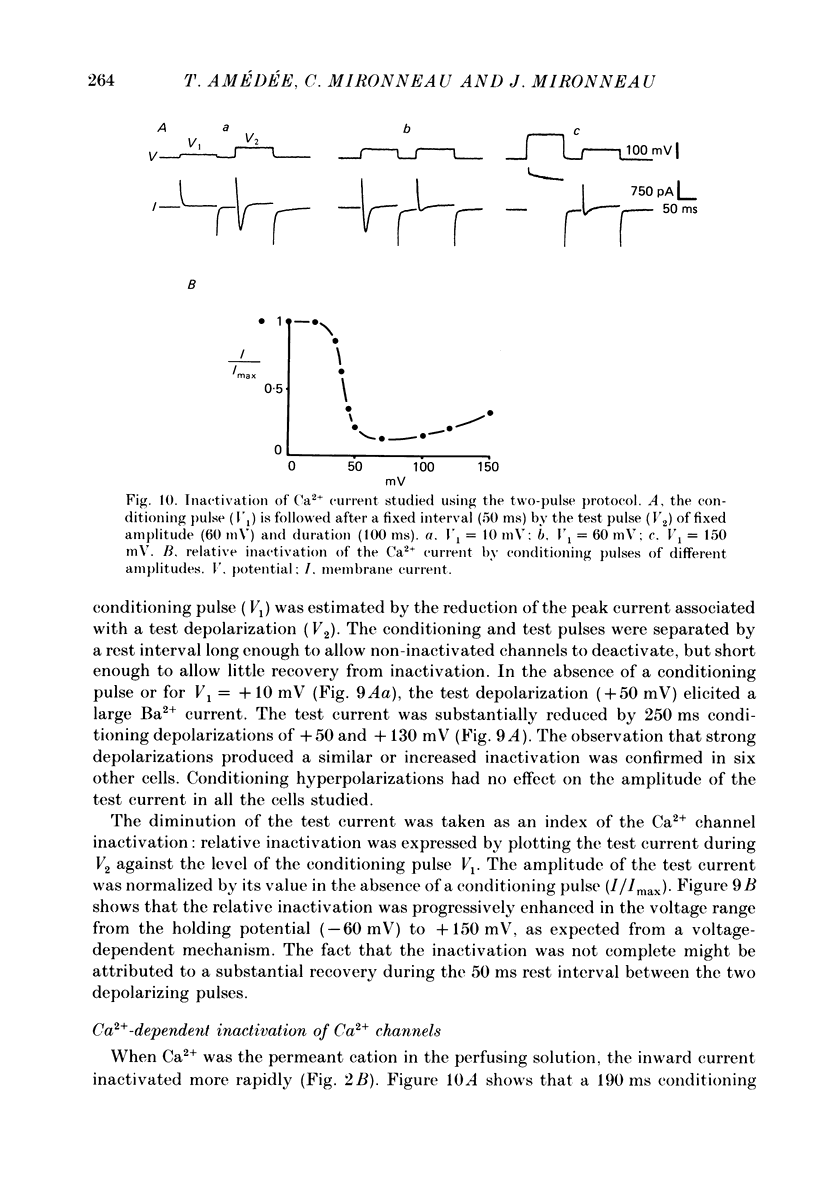
- Jmari K., Mironneau C., Mironneau J. Inactivation of calcium channel current in rat uterine smooth muscle: evidence for calcium- and voltage-mediated mechanisms. J Physiol. 1986 Nov;380:111–126. doi: 10.1113/jphysiol.1986.sp016275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jmari K., Mironneau C., Mironneau J. Selectivity of calcium channels in rat uterine smooth muscle: interactions between sodium, calcium and barium ions. J Physiol. 1987 Mar;384:247–261. doi: 10.1113/jphysiol.1987.sp016453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. Evidence for two distinct calcium channels in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1986 Nov;407(5):566–568. doi: 10.1007/BF00657519. [DOI] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
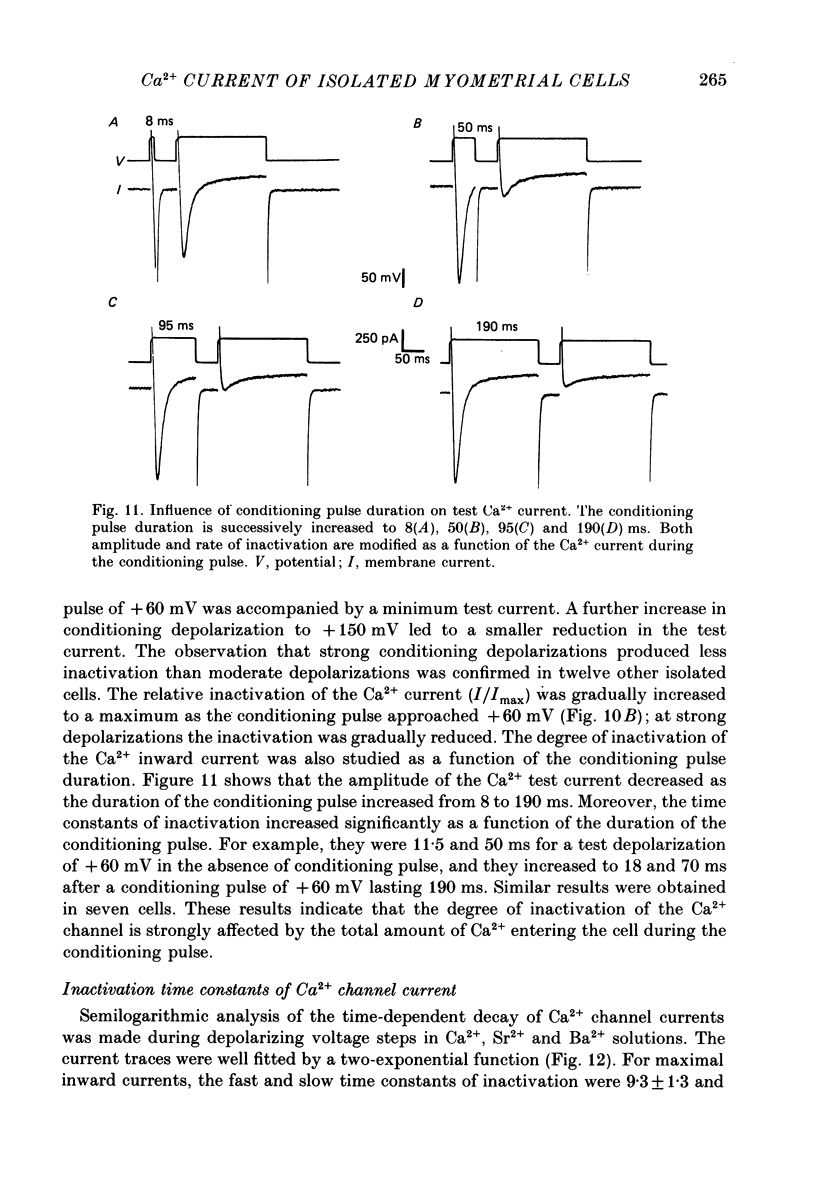
- Mironneau J., Eugene D., Mironneau C. Sodium action potentials induced by calcium chelation in rat uterine smooth muscle. Pflugers Arch. 1982 Nov 11;395(3):232–238. doi: 10.1007/BF00584815. [DOI] [PubMed] [Google Scholar]
- Mironneau J., Savineau J. P., Mironneau C. Fast outward current controlling electrical activity in rat uterine smooth muscle during gestation. J Physiol (Paris) 1981 Mar;77(8):851–859. [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
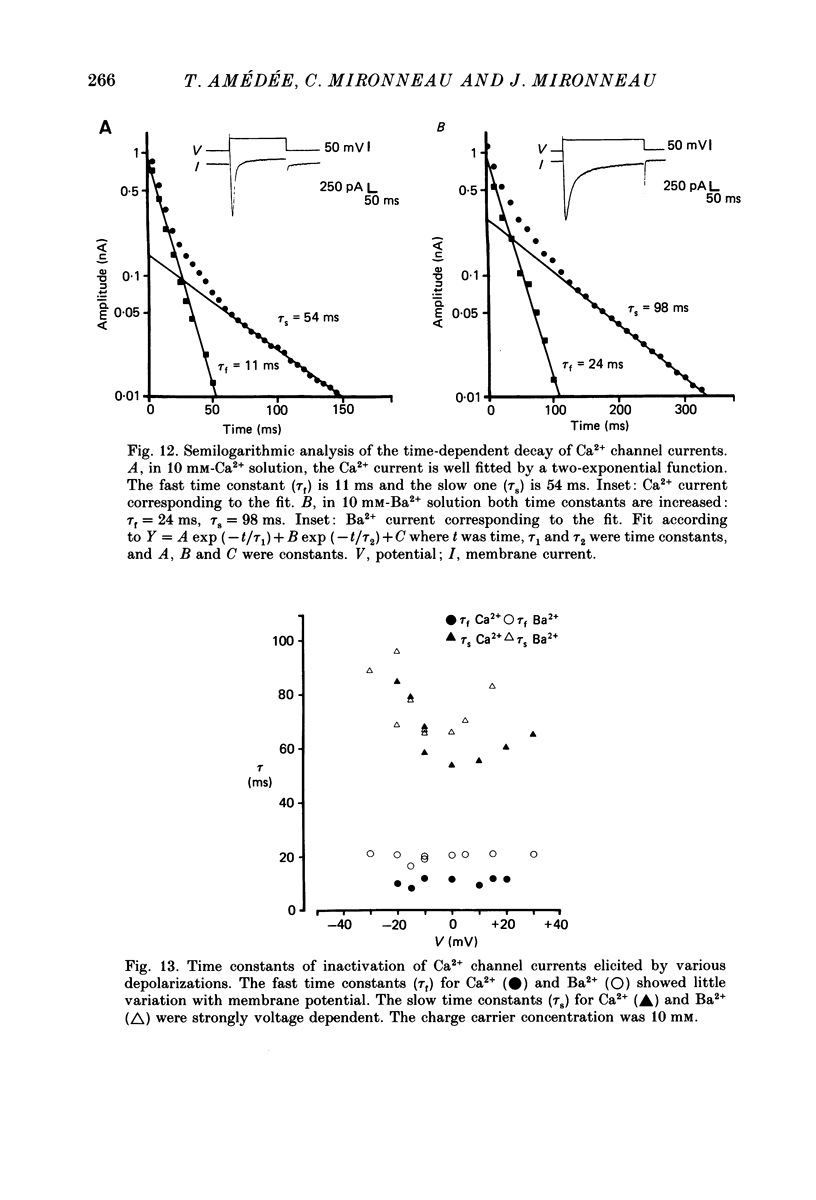
- Sturek M., Hermsmeyer K. Calcium and sodium channels in spontaneously contracting vascular muscle cells. Science. 1986 Jul 25;233(4762):475–478. doi: 10.1126/science.2425434. [DOI] [PubMed] [Google Scholar]
- Worley J. F., 3rd, Deitmer J. W., Nelson M. T. Single nisoldipine-sensitive calcium channels in smooth muscle cells isolated from rabbit mesenteric artery. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5746–5750. doi: 10.1073/pnas.83.15.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]


