Abstract
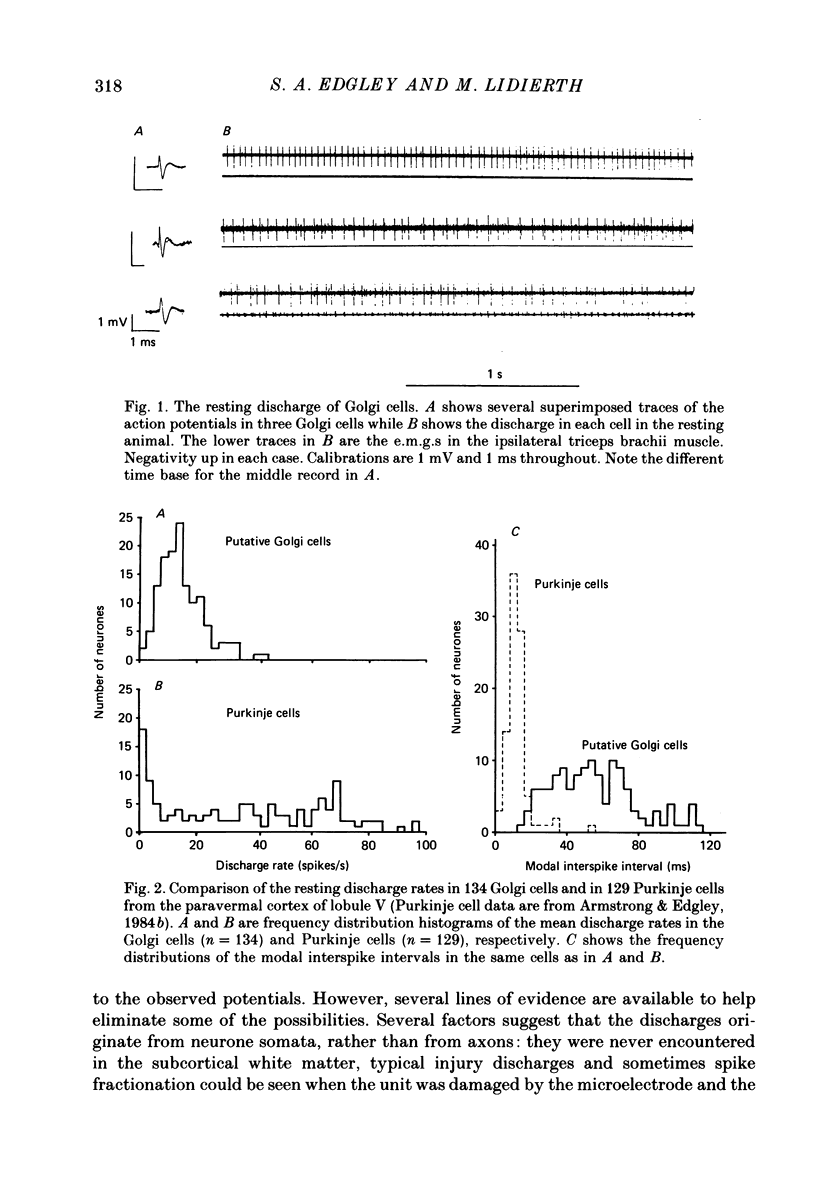
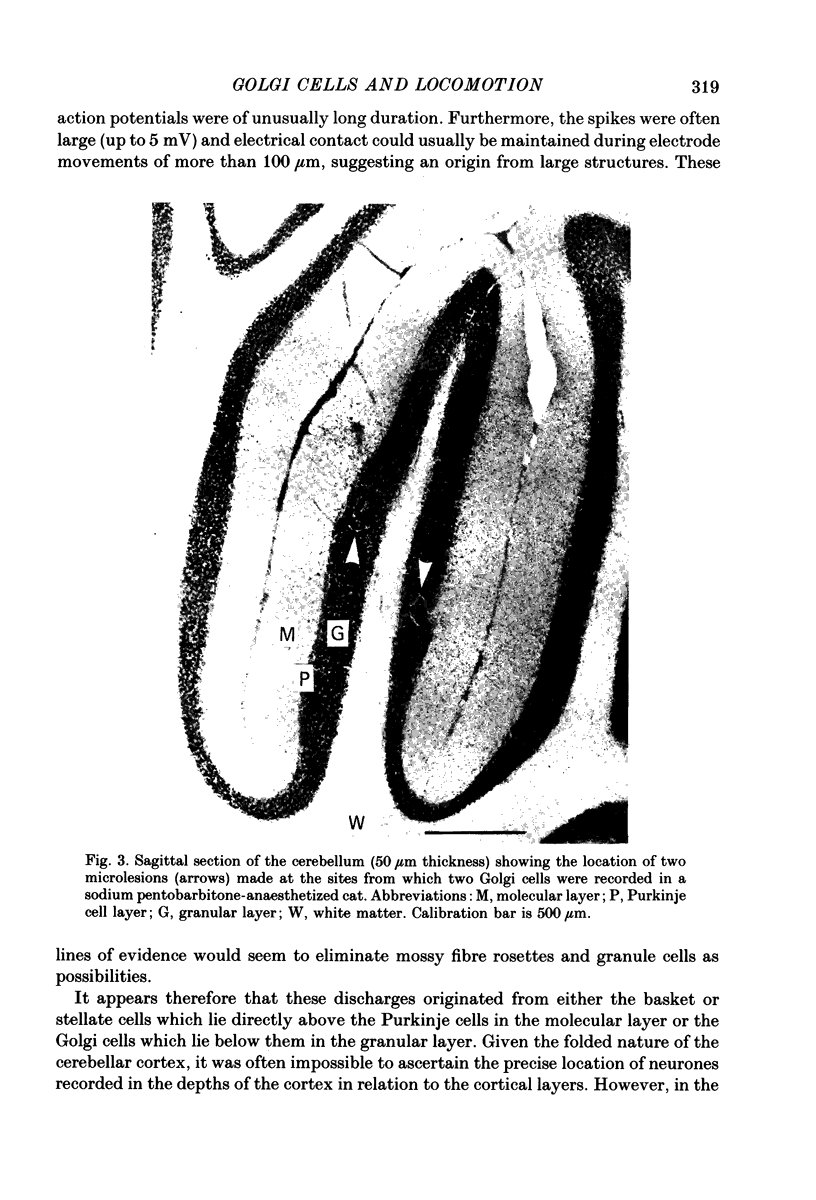
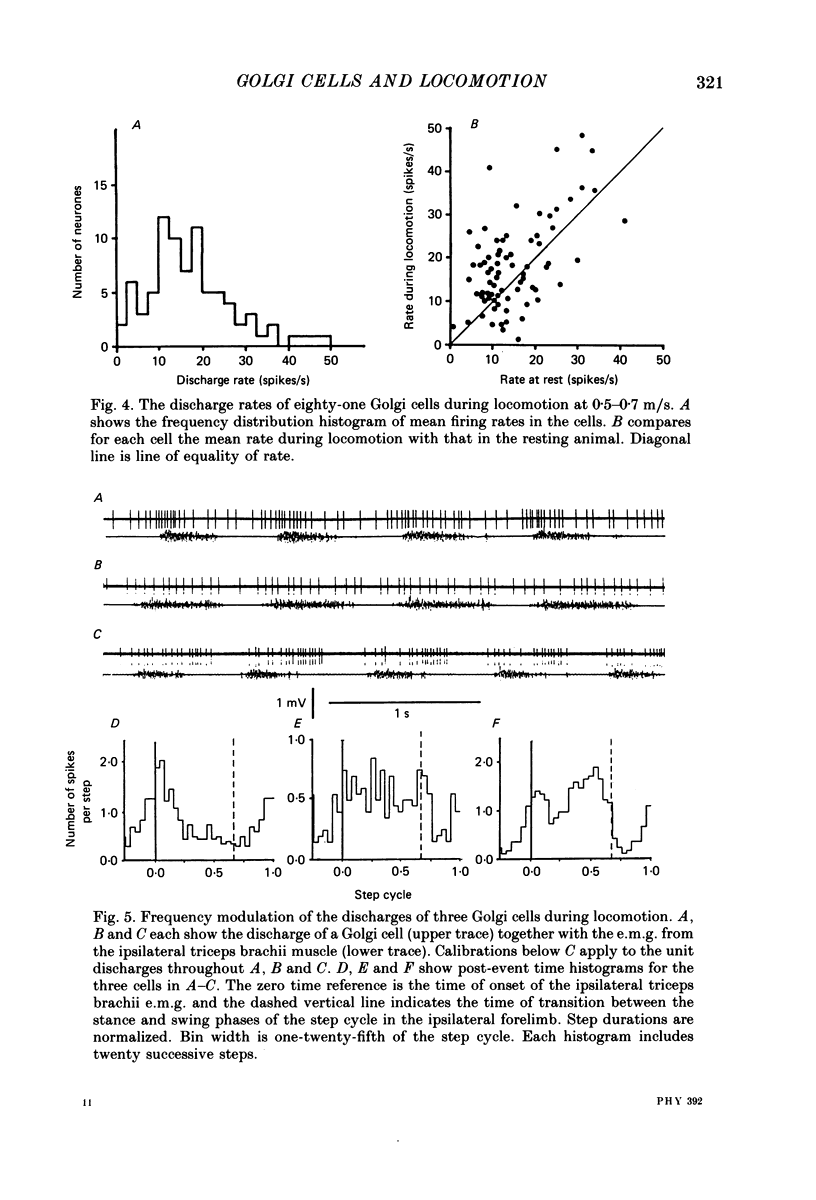
1. Extracellular recordings have been obtained from 134 units in the paravermal cortex of lobule V in the anterior lobe of the cerebellum in free-to-move cats. Each unit discharged action potentials in a characteristic pattern with no complex spikes. Previous investigators have described such discharges and have assigned them to Golgi cells. We provide further evidence to support this identification. 2. In cats which were sitting quietly, each putative Golgi cell discharged tonically at a low rate (overall mean (+/- S.D.) was 14.5 +/- 7.3 spikes/s). Eighty-one of the neurones were also recorded during steady walking on a moving belt and the discharge rate then averaged 17.7 (S.D. +/- 19.4) spikes/s. 3. All eighty-one putative Golgi cells discharged rhythmically during locomotion. Many different patterns of discharge were seen but as a population the neurons were most active in early stance and at the onset of the swing phase of the step cycle in the ipsilateral forelimb. 4. Over a range of different walking speeds (0.5-0.9 m/s) and of different uphill inclines (0-25 deg), putative Golgi cells discharged with similar patterns and rates, despite large changes in the vigour of the locomotor movements and in the amplitude of limb muscle electromyograms. 5. Groups of putative Golgi cells and of Purkinje cells recorded from restricted cortical regions produced discharges which fluctuated approximately in parallel during locomotion. However, a comparison of the activity patterns of pairs of closely adjacent Golgi and Purkinje cells revealed a wide variety of phase relations. The possible role of Golgi cells in determining the Purkinje cell output is discussed in the light of these findings.
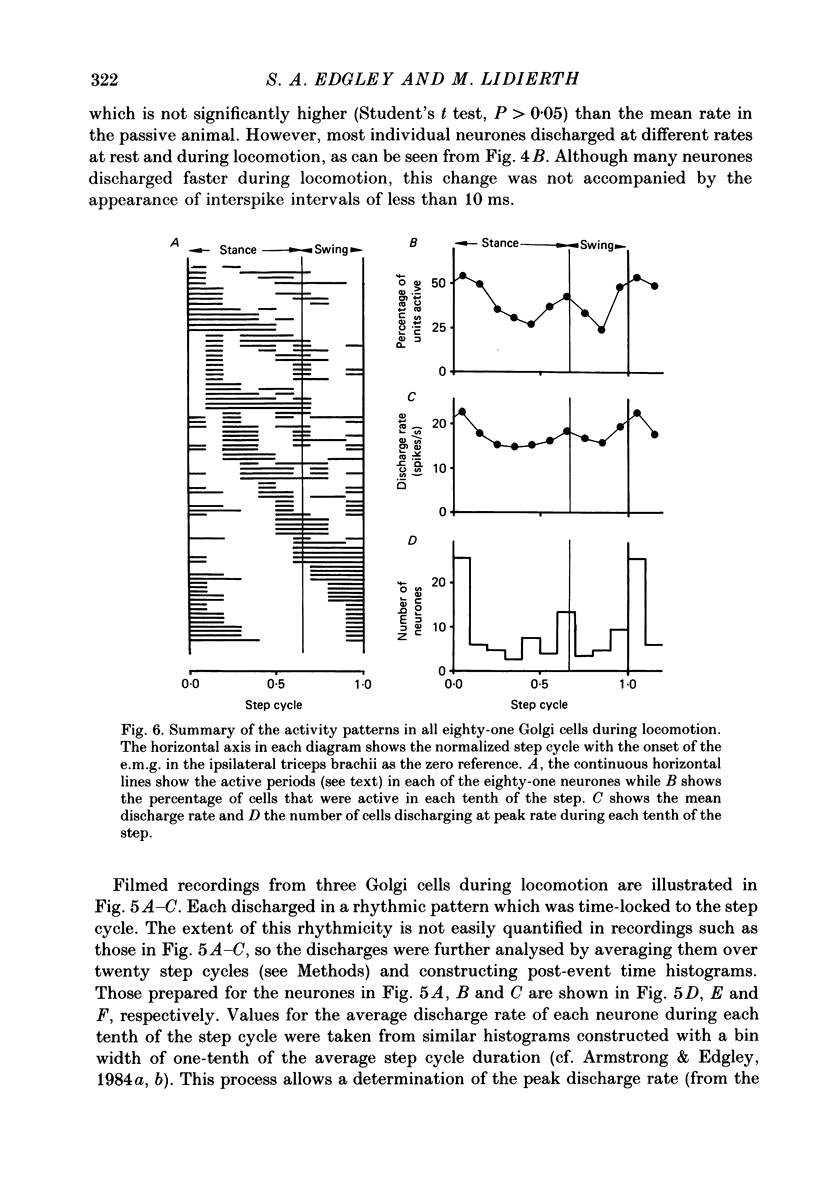
Full text
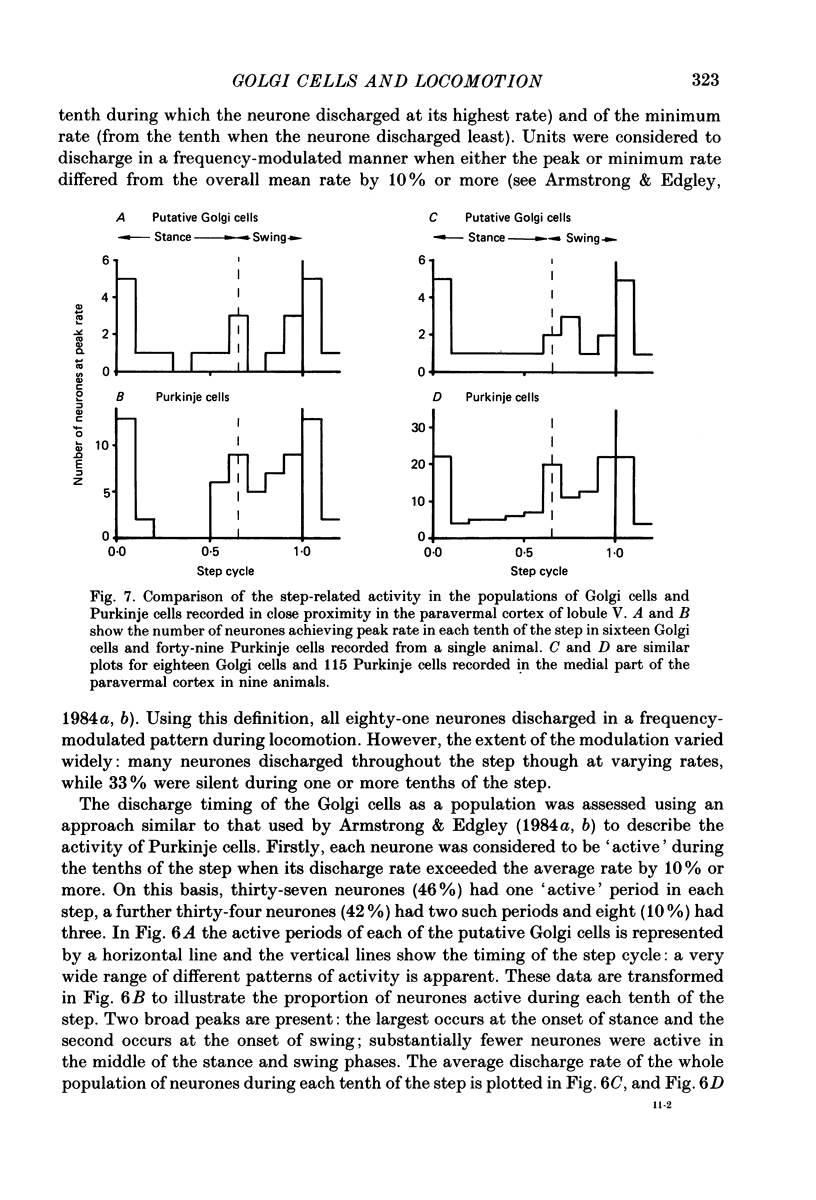
PDF




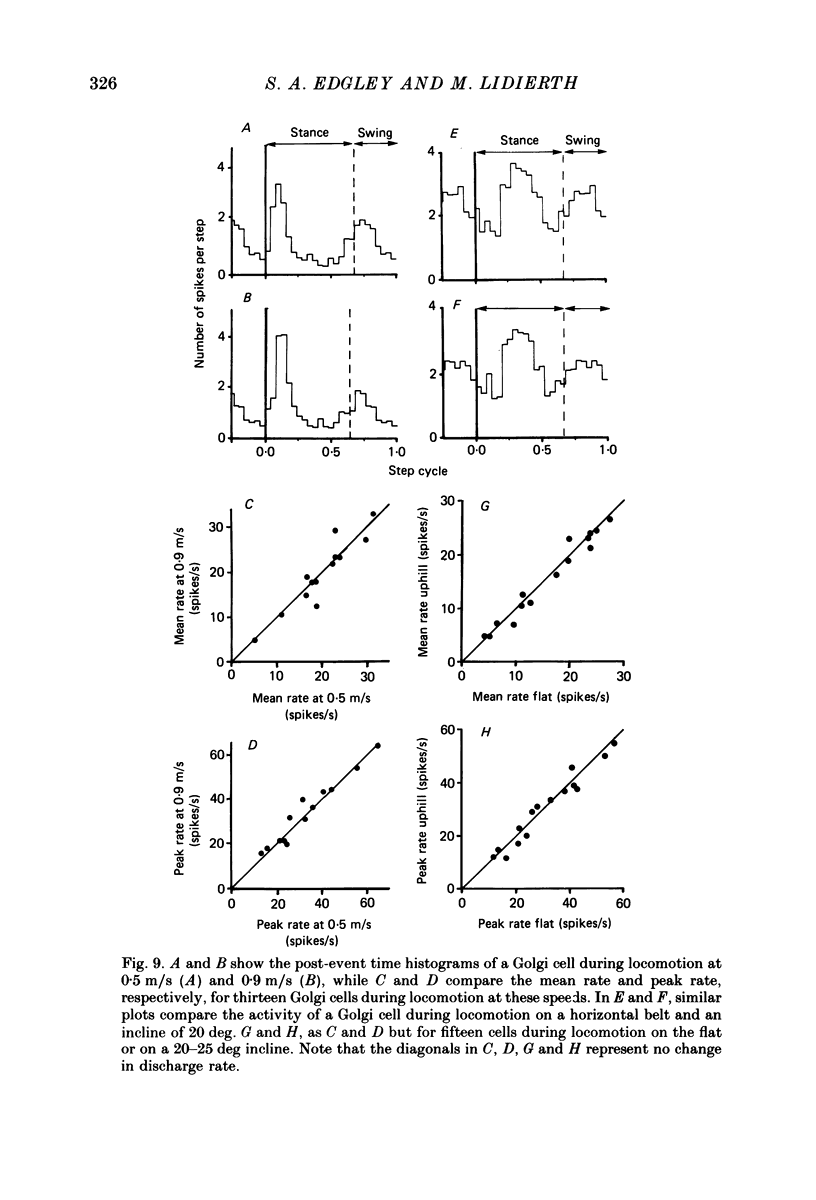






Images in this article
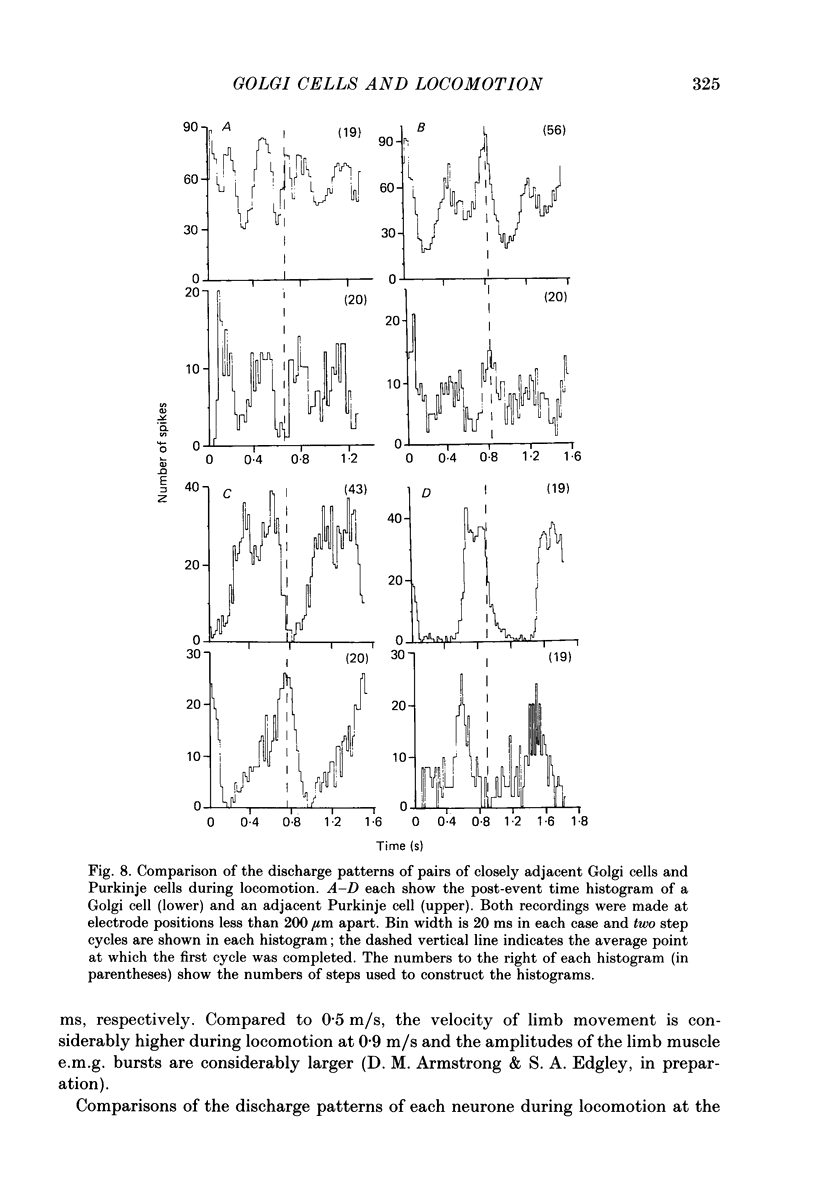
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 1984 Jul;352:403–424. doi: 10.1113/jphysiol.1984.sp015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 1984 Jun;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979 Apr;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YuI, Orlovsky G. N., Popova L. B. Activity of cerebellar Purkinje cells during fictitious scratch reflex in the cat. Brain Res. 1984 Jan 2;290(1):33–41. doi: 10.1016/0006-8993(84)90733-9. [DOI] [PubMed] [Google Scholar]
- Bower J. M., Woolston D. C. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol. 1983 Mar;49(3):745–766. doi: 10.1152/jn.1983.49.3.745. [DOI] [PubMed] [Google Scholar]
- Brand S., Dahl A. L., Mugnaini E. The length of parallel fibers in the cat cerebellar cortex. An experimental light and electron microscopic study. Exp Brain Res. 1976 Aug 27;26(1):39–58. doi: 10.1007/BF00235248. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1(1):82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979 Jul 2;36(2):201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Hámori J., Szentágothai J. Participation of Golgi neuron processes in the cerebellar glomeruli: an electron microscope study. Exp Brain Res. 1966;2(1):35–48. doi: 10.1007/BF00234359. [DOI] [PubMed] [Google Scholar]
- Lange W. Regional differences in the distribution of golgi cells in the cerebellar cortex of man and some other mammals. Cell Tissue Res. 1974;153(2):219–226. doi: 10.1007/BF00226610. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Fuchs A. F. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. II. Mossy fiber firing patterns during horizontal head rotation and eye movement. J Neurophysiol. 1978 May;41(3):764–777. doi: 10.1152/jn.1978.41.3.764. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. Proceedings: Iron-plated tungsten micro-electrodes for tip marking. J Physiol. 1974 Sep;241(2):68P–69P. [PubMed] [Google Scholar]
- Miles F. A., Fuller J. H., Braitman D. J., Dow B. M. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980 May;43(5):1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Nahvi M. J., Woody C. D., Tzebelikos E., Ribak C. E. Electrophysiologic characterization of morphologically identified neurons in the cerebellar cortex of awake cats. Exp Neurol. 1980 Feb;67(2):368–376. doi: 10.1016/0014-4886(80)90236-8. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Magyar P., Szentágothai J. Quantitative histological analysis of the cerebellar cortex in the cat. II. Cell numbers and densities in the granular layer. Brain Res. 1971 Sep 10;32(1):15–30. doi: 10.1016/0006-8993(71)90152-1. [DOI] [PubMed] [Google Scholar]
- Schulman J. A., Bloom F. E. Golgi cells of the cerebellum are inhibited by inferior olive activity. Brain Res. 1981 Apr 6;210(1-2):350–355. doi: 10.1016/0006-8993(81)90908-2. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Minoda K., Oda Y. Simple and complex spike activities of Purkinje cells during locomotion in the cerebellar vermal zones of decerebrate cats. Exp Brain Res. 1981;41(3-4):292–300. doi: 10.1007/BF00238886. [DOI] [PubMed] [Google Scholar]



