Abstract
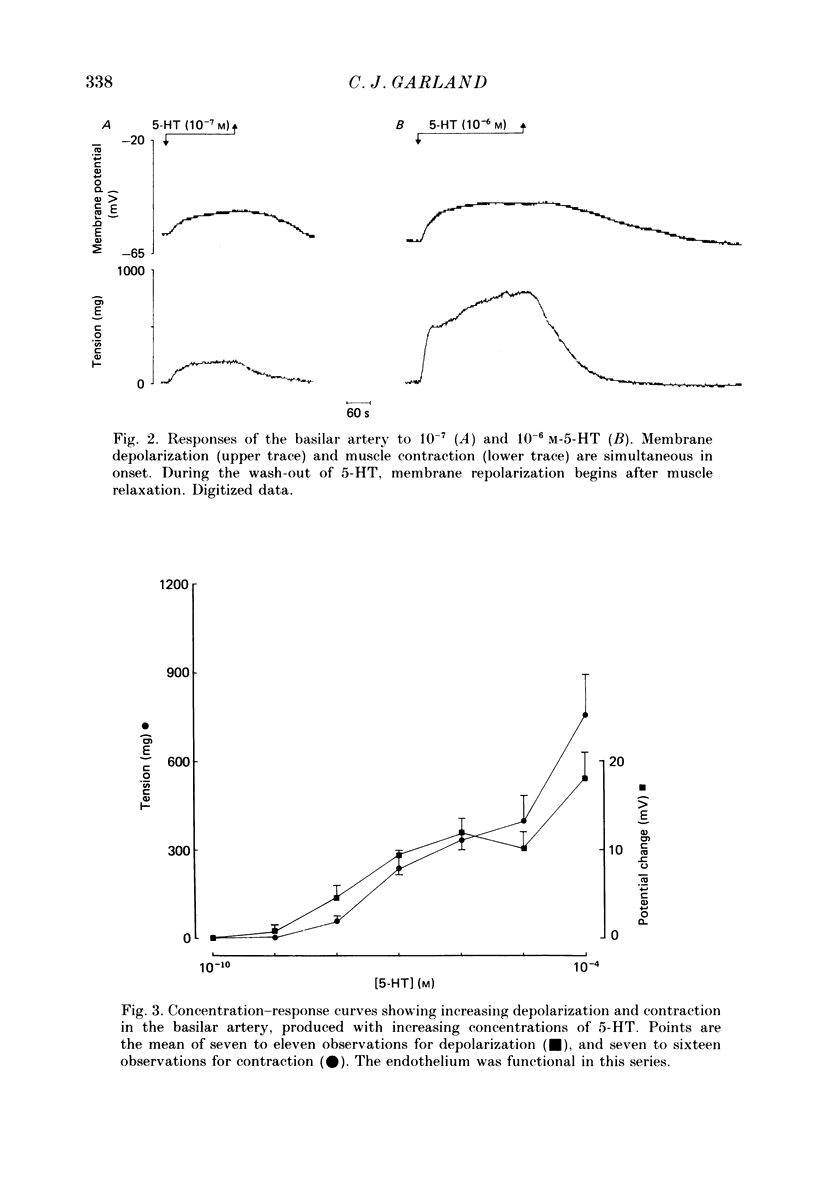
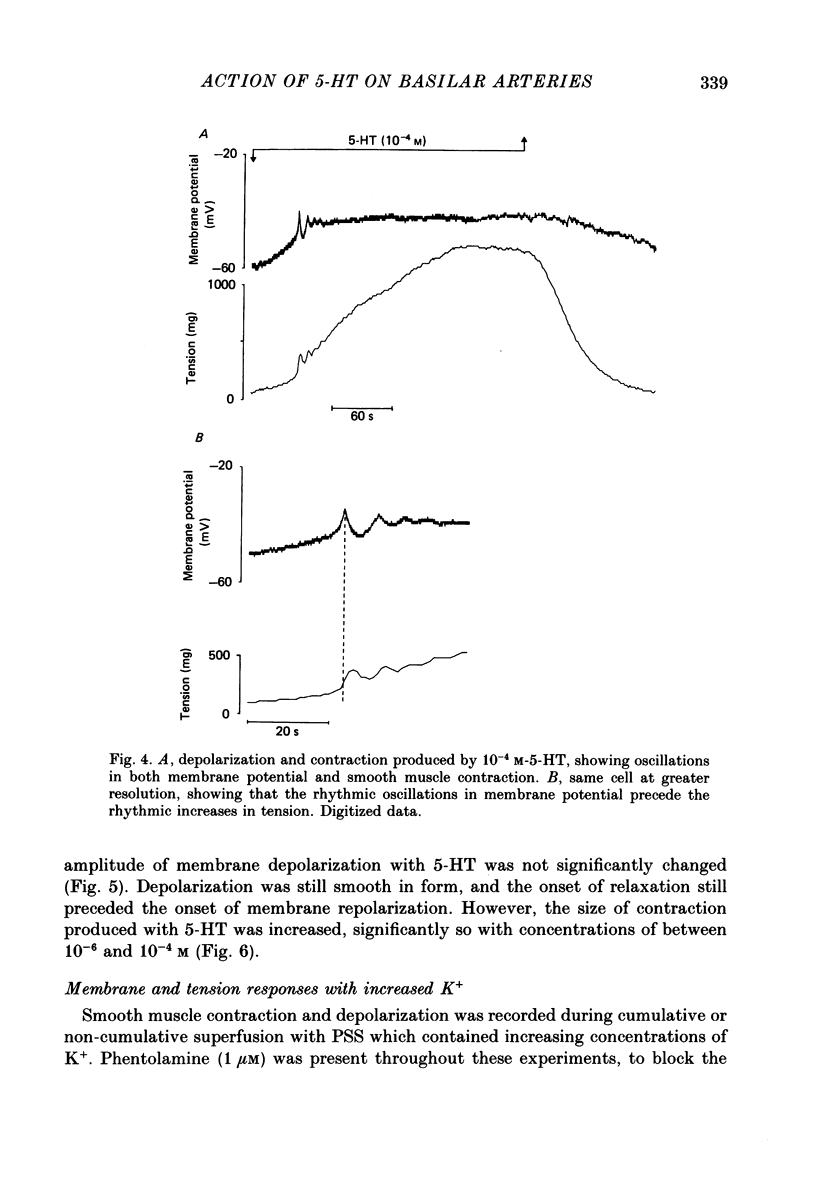
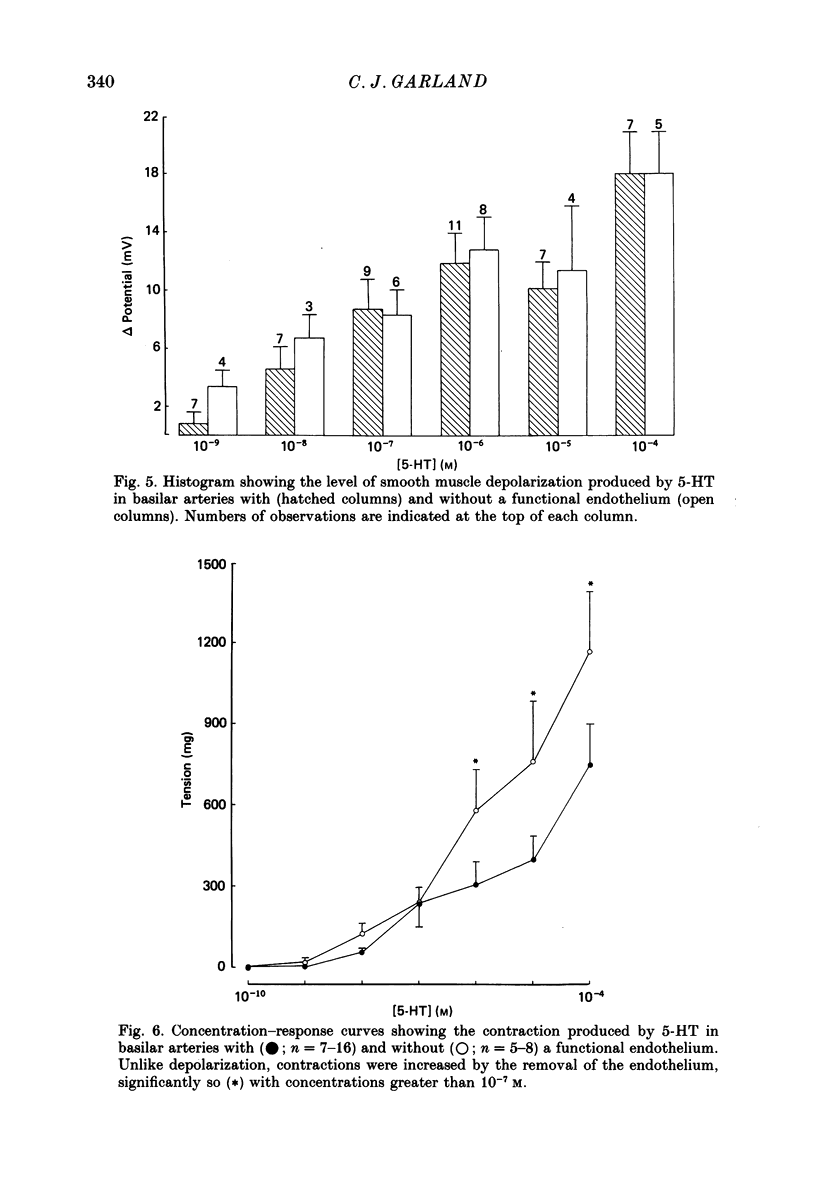
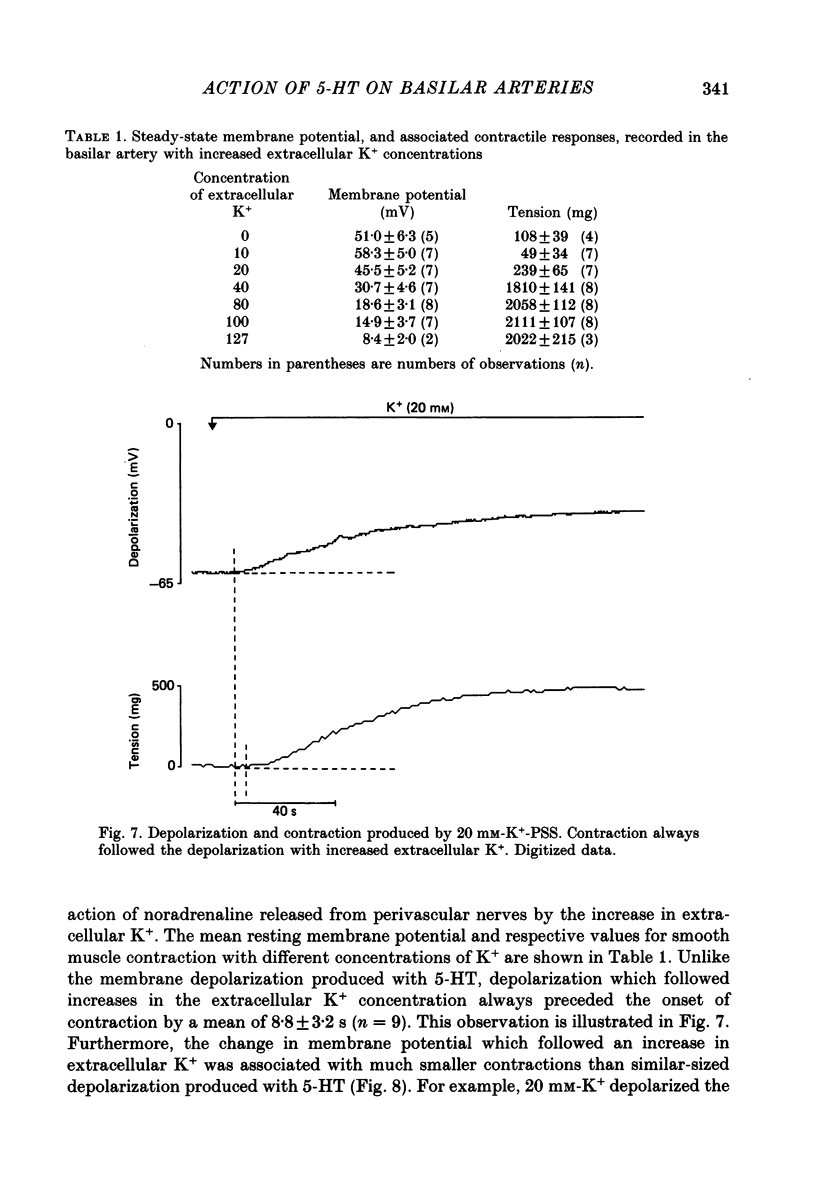
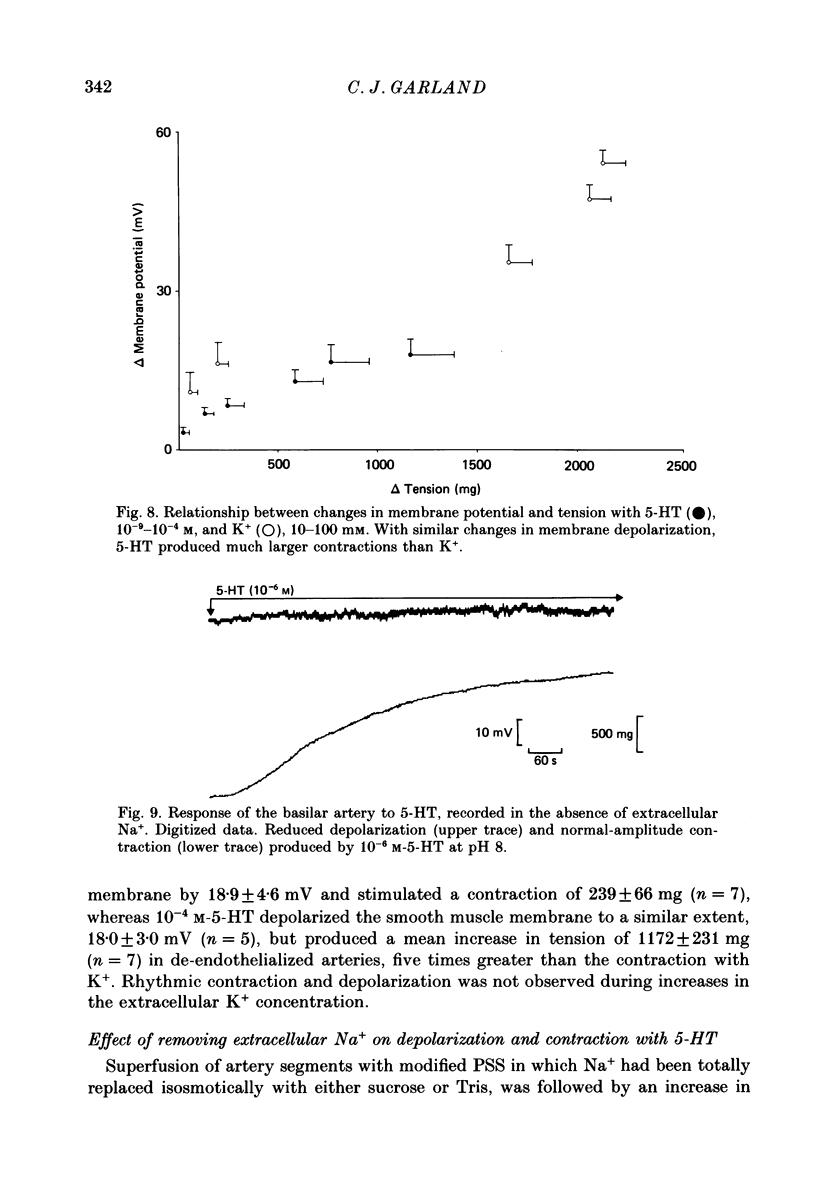
1. 5-Hydroxytryptamine (5-HT, 10(-9)-10(-4) M) depolarized and contracted smooth muscle cells (resting potential: -69.1 +/- 0.9 mV, n = 112) in isolated cylindrical segments of the rabbit basilar artery. 2. Simultaneous measurement of membrane potential and wall tension (n = 43, thirteen vessels) showed that the onset of 5-HT-induced depolarization coincided with the onset of smooth muscle contraction in the majority of cells studied. In addition, the onset of relaxation which followed the wash-out of 5-HT always preceded the onset of membrane repolarization by 52 +/- 8 s (n = 14). 3. In 30% of smooth muscle cells exposed to concentrations of 5-HT greater than 10(-6) M, fast rhythmic depolarizations (amplitude 10-20 mV) were superimposed on the developing depolarization. Rhythmic membrane depolarization was always followed by rhythmic smooth muscle contraction, which peaked 2-4 s after the peak of the fast depolarization. 4. Muscle contraction, but not depolarization, produced with concentrations of 5-HT greater than 10(-7) M, was significantly increased by the removal of intimal-endothelial cells. 5. Smooth muscle depolarization recorded in the presence of increased extracellular K+ (greater than 5.2 mM) preceded the onset of smooth muscle contraction. For a similar change in membrane potential produced with either increased extracellular K+ or 5-HT, the corresponding increase in arterial wall tension was always greater with 5-HT. 6. The depolarization and contraction induced by 5-HT was markedly reduced or abolished if extracellular Na+ was totally replaced, isosmotically, with either sucrose or Tris at pH 7.4. Normal-sized contraction, but not depolarization, was recorded with 5-HT in Na+-free Tris solution at pH 8. 7. These observations suggest that 5-HT-stimulated contraction in cerebrovascular smooth muscle is largely a result of mechanisms other than depolarization of the smooth muscle cell membrane which it produces. However, high concentrations of 5-HT (greater than 10(-6) M) can stimulate additional depolarization, which has a faster time course and rhythmic nature. Discrete depolarizations of this type are responsible for initiating additional, phasic smooth muscle contractions.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhle Y. S., Vane J. R. Pharmacokinetic function of the pulmonary circulation. Physiol Rev. 1974 Oct;54(4):1007–1045. doi: 10.1152/physrev.1974.54.4.1007. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Calcium metabolism in vascular smooth muscle. Br Med Bull. 1986 Oct;42(4):421–429. doi: 10.1093/oxfordjournals.bmb.a072161. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. An electrophysiological study of alpha-adrenoceptor mediated excitation-contraction coupling in the smooth muscle cells of the rat saphenous vein. Br J Pharmacol. 1985 Jan;84(1):265–271. [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Yamaguchi T. Membrane potential-dependent and-independent tension in the canine tracheal muscle. J Pharmacol Exp Ther. 1977 May;201(2):276–284. [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983 Oct 13;305(5935):627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Shepherd J. T., Vanhoutte P. M. 5-Hydroxytryptamine can mediate endothelium-dependent relaxation of coronary arteries. Am J Physiol. 1983 Dec;245(6):H1077–H1080. doi: 10.1152/ajpheart.1983.245.6.H1077. [DOI] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Degueurce A., Duverger D., MacKenzie E. T., Scatton B. Central serotonergic nerves project to the pial vessels of the brain. Nature. 1983 Nov 3;306(5938):55–57. doi: 10.1038/306055a0. [DOI] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Kuriyama H. Nicardipine actions on smooth muscle cells and neuromuscular transmission in the guinea-pig basilar artery. J Pharmacol Exp Ther. 1983 May;225(2):447–455. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland C. J. Endothelial cells and the electrical and mechanical responses of the rabbit coronary artery to 5-hydroxytryptamine. J Pharmacol Exp Ther. 1985 Apr;233(1):158–162. [PubMed] [Google Scholar]
- Graham J. M., Keatinge W. R. Responses of inner and outer muscle of the sheep carotid artery to injury. J Physiol. 1975 May;247(2):473–482. doi: 10.1113/jphysiol.1975.sp010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S. G., Lincoln J., Burnstock G. Serotonin as a neurotransmitter in cerebral arteries. Brain Res. 1982 Sep 16;247(2):388–392. doi: 10.1016/0006-8993(82)91266-5. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Waters A. Electromechanical coupling in feline basilar artery in response to serotonin. Eur J Pharmacol. 1983 Sep 16;93(1-2):95–100. doi: 10.1016/0014-2999(83)90034-1. [DOI] [PubMed] [Google Scholar]
- Harper M. A., MacKenzie E. T. Cerebral circulatory and metabolic effects of 5-hydroxytryptamine in anesthetized baboons. J Physiol. 1977 Oct;271(3):721–733. doi: 10.1113/jphysiol.1977.sp012022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEATINGE W. R. MECHANISM OF ADRENERGIC STIMULATION OF MAMMALIAN ARTERIES AND ITS FAILURE AT LOW TEMPERATURES. J Physiol. 1964 Nov;174:184–205. doi: 10.1113/jphysiol.1964.sp007481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Mekata F. Different electrical responses of outer and inner muscle of rabbit carotid artery to noradrenaline and nerves. J Physiol. 1984 Jan;346:589–598. doi: 10.1113/jphysiol.1984.sp015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulds R. F., Iwanov V., Medcalf R. L. The effects of platelet-derived contractile agents on human digital arteries. Clin Sci (Lond) 1984 Apr;66(4):443–451. doi: 10.1042/cs0660443. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A., Korsgaard N. Potentiating and depressive effects of ouabain and potassium-free solutions on rat mesenteric resistance vessels. Circ Res. 1982 Oct;51(4):514–524. doi: 10.1161/01.res.51.4.514. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- RAND M., REID G. Source of 'serotonin' in serum. Nature. 1951 Sep 1;168(4270):385–385. doi: 10.1038/168385b0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Richardson B. P., Engel G., Donatsch P., Stadler P. A. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985 Jul 11;316(6024):126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Small R., Macarak E., Fisher A. B. Production of 5-hydroxyindoleacetic acid from serotonin by cultured endothelial cells. J Cell Physiol. 1977 Feb;90(2):225–231. doi: 10.1002/jcp.1040900208. [DOI] [PubMed] [Google Scholar]
- WAUGH W. H. Adrenergic stimulation of depolarized arterial muscle. Circ Res. 1962 Aug;11:264–276. doi: 10.1161/01.res.11.2.264. [DOI] [PubMed] [Google Scholar]


