Abstract
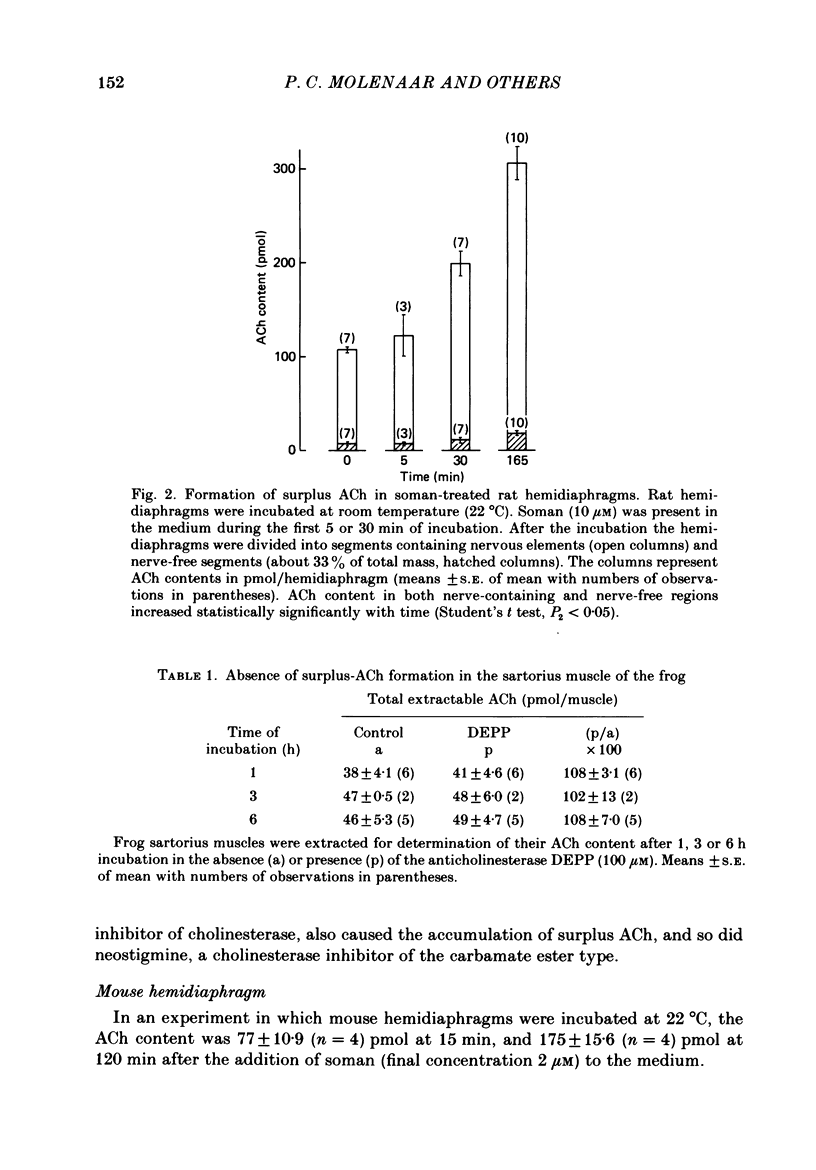
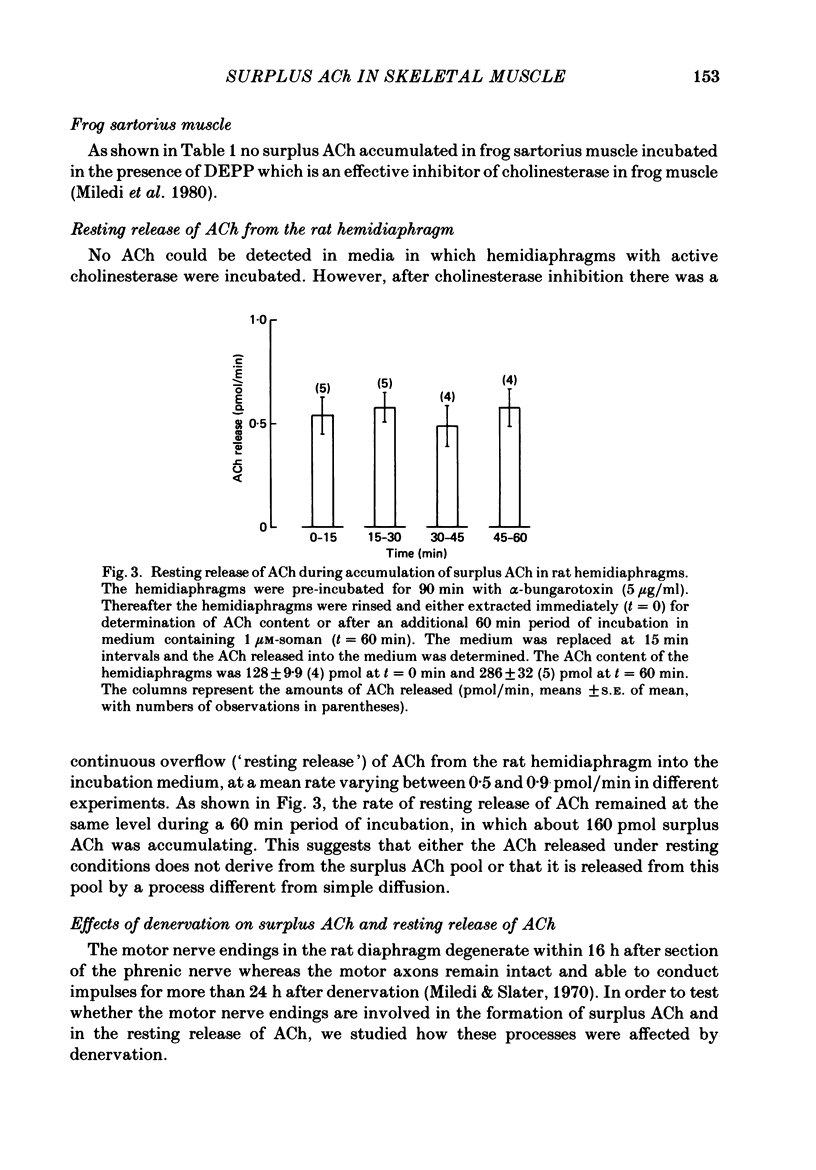
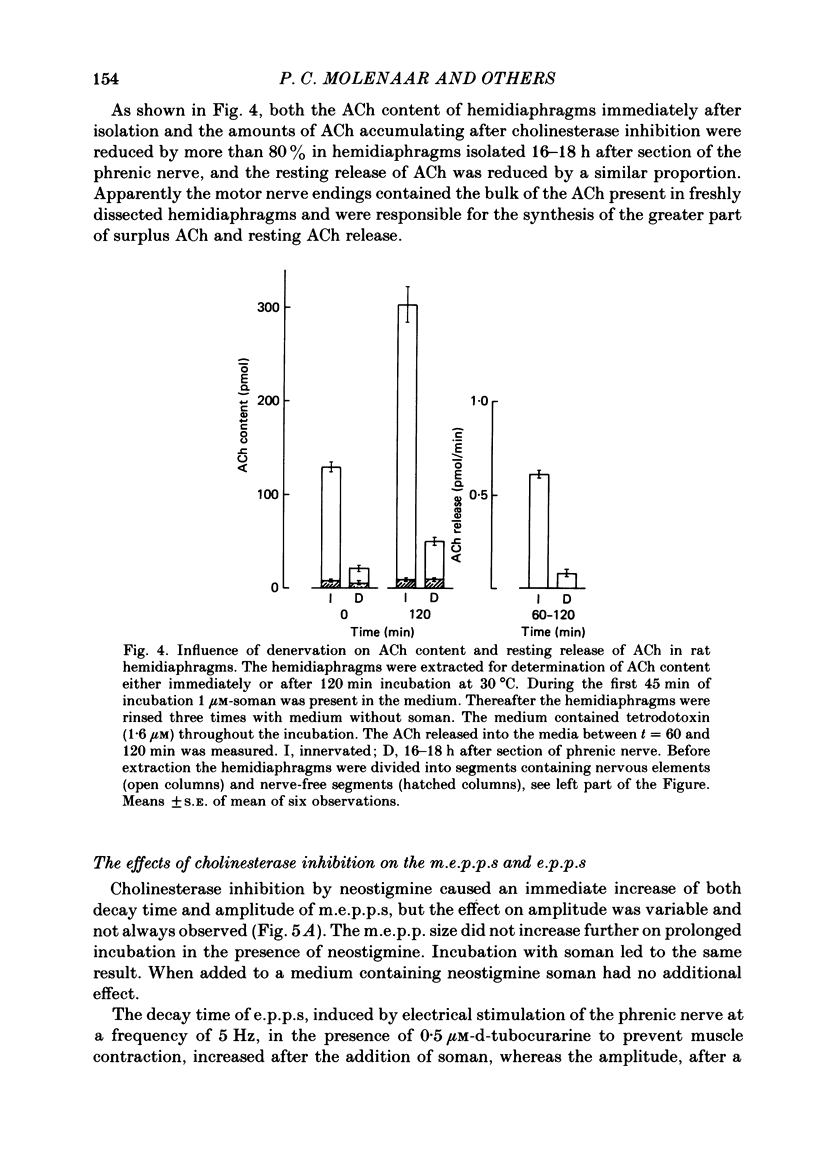
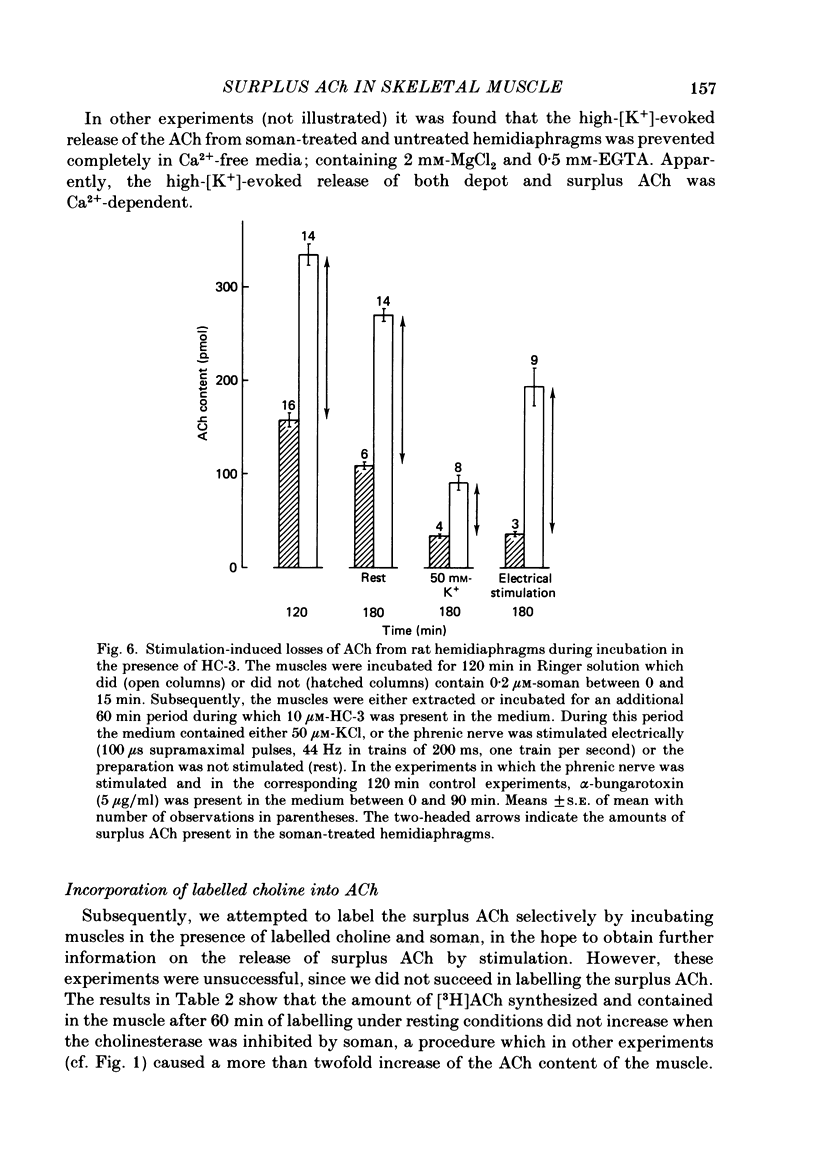
1. Skeletal muscles from rat, mouse and frog were incubated under different conditions and the amounts of acetylcholine (ACh) extractable from the tissue and released into the medium were determined by mass fragmentography. In some experiments measurements were made of the amounts of ACh ('bound' ACh) surviving in a muscle homogenate to which an excess of acetylcholinesterase had been added. In other experiments the membrane potentials, end-plate potentials (e.p.p.s), and miniature end-plate potentials (m.e.p.p.s) were studied. 2. During incubation in Ringer medium the ACh content of the rat hemidiaphragm usually did not change, but after inhibition of cholinesterase by soman the ACh content rose gradually from about 100 to 150 pmol to a plateau of about 400 pmol after 4 h. A similar formation of 'surplus ACh' after cholinesterase inhibition was found in the mouse diaphragm, but not in the frog sartorius muscle. 3. Surplus ACh accumulated predominantly in the end-plate region of the rat diaphragm. In muscles, 16-18 h after in vivo denervation, the capacity to form surplus ACh was decreased by more than 80%. 4. The amount of ACh diffusing from the resting hemidiaphragm into the incubation medium ('resting release') varied between 0.5 and 0.9 pmol min-1 in different experiments; it remained at the same level during accumulation of surplus ACh. It was reduced by more than 80% 16-18 h after denervation. 5. The amplitude of m.e.p.p.s and e.p.p.s did not increase while surplus ACh was accumulating. 6. Incubation of hemidiaphragms in Ringer solution containing [3H]choline caused the formation of [3H]ACh. Additional amounts of [3H]choline were incorporated into ACh when the nerve was stimulated for 60 min. However, incubation in the presence of soman (3,3-dimethyl-2-butylmethylphosphonofluoridate), in the absence of stimulation, did not cause an increase of the [3H]ACh content of the muscles. 7. From hemidiaphragms with active cholinesterase about 120 pmol ACh was lost after prolonged nerve stimulation or incubation with 50 mM-KCl in the presence of hemicholinium-3, and about 35 pmol remained in the tissue. In soman-treated muscles, containing surplus ACh, about as much ACh was released by nervous stimulation as from untreated hemidiaphragms, and much more ACh remained unreleased. 8. Transection of the muscle at both sides of the end-plate or incubation of intact muscles in the presence of 50 mM-KCl depolarized the muscle fibres to -35 and -31 mV, respectively. Surplus ACh was partially released by 50 mM-KCl, but not by muscle transection.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamic S. Accumulation of acetylcholine by the rat diaphragm. Biochem Pharmacol. 1970 Aug;19(8):2445–2451. doi: 10.1016/0006-2952(70)90269-8. [DOI] [PubMed] [Google Scholar]
- Collier B., Katz H. S. The synthesis, turnover and release of surplus acetylcholine in a sympathetic ganglion. J Physiol. 1971 May;214(3):537–552. doi: 10.1113/jphysiol.1971.sp009447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. A., Saunders N. R. An outflow of acetylcholine from normal and regenerating ventral roots of the cat. J Physiol. 1974 Jul;240(1):15–32. doi: 10.1113/jphysiol.1974.sp010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P., Forrester T. The effect of curare on the release of acetylcholine from mammalian motor nerve terminals and an estimate of quantum content. J Physiol. 1975 Sep;251(1):131–144. doi: 10.1113/jphysiol.1975.sp011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Hurlbut W. P., Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J Cell Biol. 1978 Sep;78(3):716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M., Lesbats B., Manaranche R., Morel N. Acetylcholine release from proteoliposomes equipped with synaptosomal membrane constituents. Biochim Biophys Acta. 1983 Mar 9;728(3):438–448. doi: 10.1016/0005-2736(83)90516-3. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Manaranche R. ACh release from osmotically shocked synaptosomes refilled with transmitter. Nature. 1981 Dec 3;294(5840):474–475. doi: 10.1038/294474a0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. Diffusion of acetylcholine in agar gels and in the isolated rat diaphragm. J Physiol. 1960 Oct;153:562–572. doi: 10.1113/jphysiol.1960.sp006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Does the motor nerve impulse evoke 'non-quantal' transmitter release? Proc R Soc Lond B Biol Sci. 1981 May 7;212(1186):131–137. doi: 10.1098/rspb.1981.0029. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Kuba K., Albuquerque E. X., Barnard E. A. Diisopropylfluorophosphate: suppression of ionic conductance of the cholinergic receptor. Science. 1973 Aug 31;181(4102):853–856. doi: 10.1126/science.181.4102.853. [DOI] [PubMed] [Google Scholar]
- Lang B., Molenaar P. C., Newsom-Davis J., Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome in mice: decreased rates of resting and evoked release of acetylcholine from skeletal muscle. J Neurochem. 1984 Mar;42(3):658–662. doi: 10.1111/j.1471-4159.1984.tb02733.x. [DOI] [PubMed] [Google Scholar]
- Meeter E. Desensitization of the end-plate membrane following cholinesterase inhibition, an adjustment to a new working situation. Acta Physiol Pharmacol Neerl. 1969 Jun;15(2):243–258. [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978 Apr 13;272(5654):641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. An analysis of acetylcholine in frog muscle by mass fragmentography. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):285–297. doi: 10.1098/rspb.1977.0071. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Free and bound acetylcholine in frog muscle. J Physiol. 1982 Dec;333:189–199. doi: 10.1113/jphysiol.1982.sp014448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L., Tas J. W., van der Laaken T. Neural and non-neural acetylcholine in the rat diaphragm. Proc R Soc Lond B Biol Sci. 1982 Jan 22;214(1195):153–168. doi: 10.1098/rspb.1982.0002. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. The effect of lanthanum ions on acetylcholine in frog muscle. J Physiol. 1980 Dec;309:199–214. doi: 10.1113/jphysiol.1980.sp013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol. 1963 Jan;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. A method for determination of acetylcholine by slow pyrolysis combined with mass fragmentography on a packed capillary column. J Neurochem. 1979 Feb;32(2):407–412. doi: 10.1111/j.1471-4159.1979.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. Pitfalls in determination of acetylcholine from brain by pyrolysis-gas chromatography/mass spectrometry. J Neurochem. 1974 Dec;23(6):1295–1297. doi: 10.1111/j.1471-4159.1974.tb12230.x. [DOI] [PubMed] [Google Scholar]
- Polak R. L. The influence of drugs on the uptake of acetylcholine by slices of rat cerebral cortex. Br J Pharmacol. 1969 May;36(1):144–152. doi: 10.1111/j.1476-5381.1969.tb08311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L. T. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970 Jan;206(1):145–166. doi: 10.1113/jphysiol.1970.sp009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr, Mayer H. E., Schmid P. G. Choline acetyltransferase activity in guinea-pig heart in vitro. J Neurochem. 1974 Dec;23(6):1197–1200. doi: 10.1111/j.1471-4159.1974.tb12217.x. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M. Electron microscope radioautography as a quantitative tool in enzyme cytochemistry. I. The distribution of acetylcholinesterase at motor end plates of a vertebrate twitch muscle. J Cell Biol. 1967 Feb;32(2):379–389. doi: 10.1083/jcb.32.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER MEER C., MEETER E. The mechanism of action of anticholinesterases. II. The effect of diisopropylfluorophosphonate (DPF) in the isolated rat phrenic nervediaphragm preparation. B. Reversible effects. Acta Physiol Pharmacol Neerl. 1956 Mar;4(4):472–481. [PubMed] [Google Scholar]
- Vizi E. S., Vyskocil F. Changes in total and quantal release of acetylcholine in the mouse diaphragm during activation and inhibition of membrane ATPase. J Physiol. 1979 Jan;286:1–14. doi: 10.1113/jphysiol.1979.sp012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch F., Schmidt D. E., Dettbarn W. D. Acetylcholine, choline acetyltransferase and cholinesterases in motor and sensory nerves of the bull frog. Biochem Pharmacol. 1972 Mar 15;21(6):847–856. doi: 10.1016/0006-2952(72)90128-1. [DOI] [PubMed] [Google Scholar]
- White H. L., Wu J. C. Choline and carnitine acetyltransferases of heart. Biochemistry. 1973 Feb 27;12(5):841–846. doi: 10.1021/bi00729a009. [DOI] [PubMed] [Google Scholar]


