Abstract
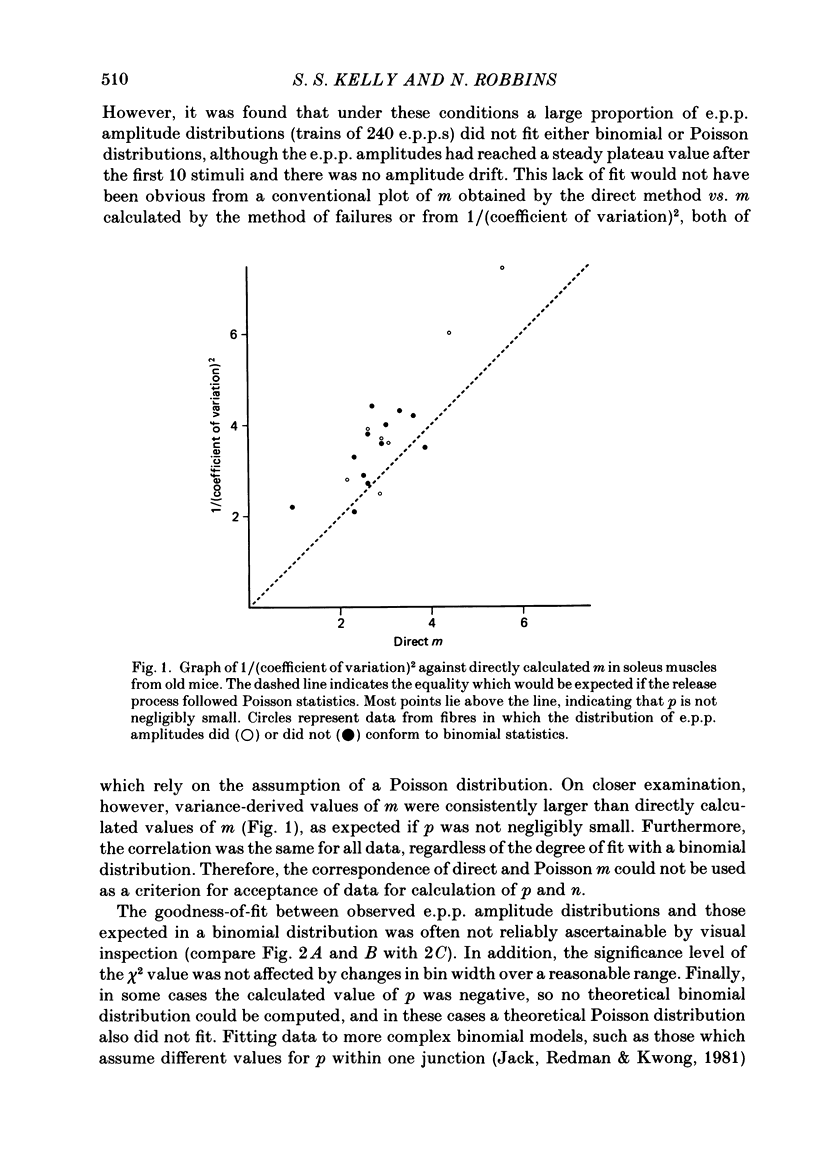
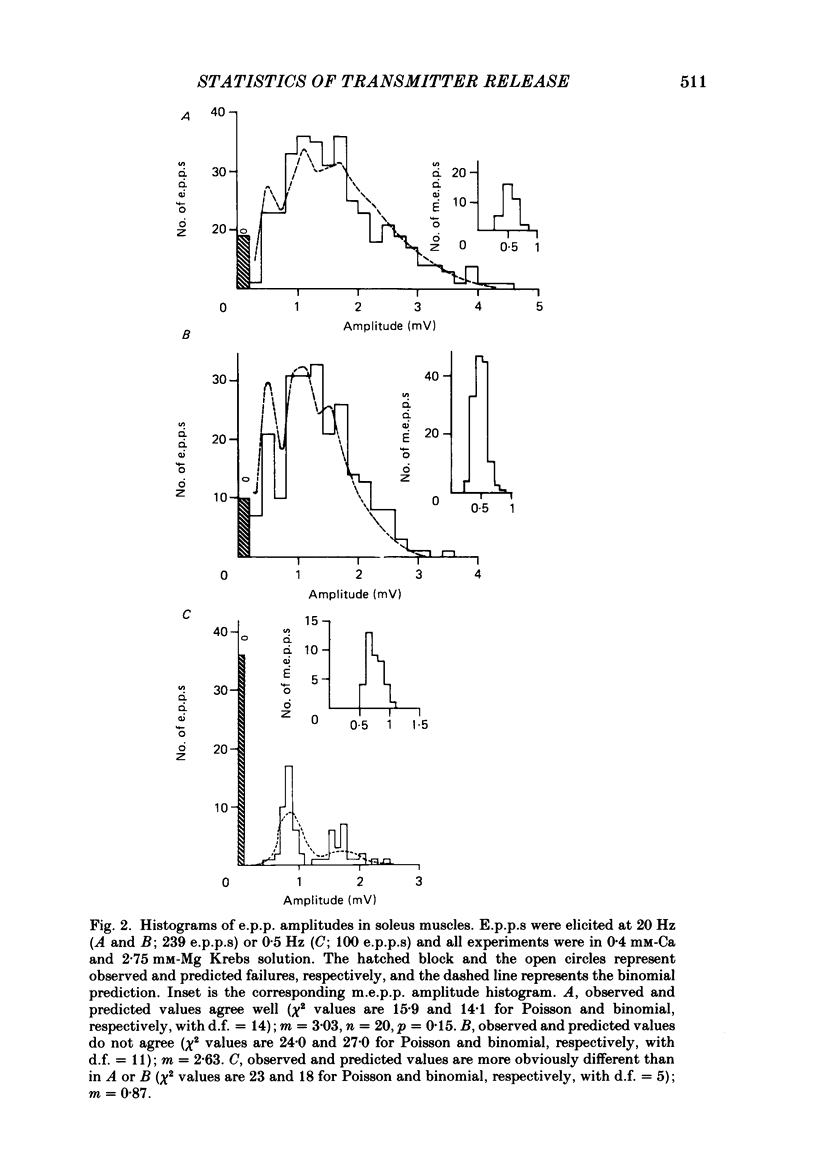
1. It was reported previously that in limb muscles of old (27-30 months) CBF-1 mice, quantal content (m) of evoked transmitter release was increased compared to that in young (9-12 months) mice. In diaphragm muscles there was no change with age. The object of the present study was to determine whether the age-related increase in transmitter release was due to increase in the binomial parameter n or the parameter p. The analysis also involved consideration of goodness-of-fit between observed and expected binomial distribution of the data. 2. Spontaneous miniature end-plate potentials (m.e.p.p.s) and evoked end-plate potentials (e.p.p.s) were recorded with intracellular techniques from soleus and diaphragm muscles bathed in low-Ca high-Mg medium. The goodness-of-fit between the observed e.p.p amplitude distribution and that expected from a binomial distribution was evaluated by chi 2 test. 3. In different muscles and at different ages, the percentage of fibres with binomial e.p.p. distributions varied from 17 to 44%, even though in all fibres there was a similar proportionality between direct quantal content and the reciprocal of the square of the coefficient of variation of e.p.p. amplitudes. In addition, apparent graphical agreement between observed and theoretical binomial e.p.p. distributions was often not substantiated by the chi 2 criterion. 4. In soleus muscles from young mice, lowering the stimulus frequency from 10 to 0.5 Hz and shortening the train length from 250 to 100 pulses increased the prevalence of binomial e.p.p. distributions, but the same result was not obtained in diaphragm or soleus muscles from old mice. If the mean amplitude of groups of 10 e.p.p.s in any train showed any drift (greater than 10%) then that train was excluded from the results. Thus, in order to make valid age comparisons, only fibres with binomial e.p.p. distributions were analysed further. 5. There was no change with age in m, n or p in diaphragm muscles, but in soleus muscles from old animals a nearly 2-fold increase in n entirely accounted for the increase in m. 6. If, as proposed by others, n represents the number of release sites, then the ageing soleus neuromuscular junction may have increased numbers or length of active zones or associated membrane components.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker B. Q., Kelly S. S., Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983 Jun;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle. J Physiol. 1974 Apr;238(1):93–107. doi: 10.1113/jphysiol.1974.sp010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Perkel D. H., Feldman M. W. Evoked neurotransmitter release: statistical effects of nonuniformity and nonstationarity. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2913–2917. doi: 10.1073/pnas.73.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavinović M. I. Voltage clamping of unparalysed cut rat diaphragm for study of transmitter release. J Physiol. 1979 May;290(2):467–480. doi: 10.1113/jphysiol.1979.sp012784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. G., Brown M. C., Keynes R. J. Postnatal growth of motor nerve terminals in muscles of the mouse. J Neurocytol. 1985 Aug;14(4):525–540. doi: 10.1007/BF01200795. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S., Robbins N. Bimodal miniature and evoked end-plate potentials in adult mouse neuromuscular junctions. J Physiol. 1984 Jan;346:353–363. doi: 10.1113/jphysiol.1984.sp015027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S., Robbins N. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. J Physiol. 1983 Oct;343:375–383. doi: 10.1113/jphysiol.1983.sp014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. P. Regeneration of the active zone at the frog neuromuscular junction. J Cell Biol. 1984 May;98(5):1685–1695. doi: 10.1083/jcb.98.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The statistics of transmitter release at chemical synapses. Int Rev Physiol. 1978;17:49–117. [PubMed] [Google Scholar]
- Miyamoto M. D. Binomial analysis of quantal transmitter release at glycerol treated frog neuromuscular junctions. J Physiol. 1975 Aug;250(1):121–142. doi: 10.1113/jphysiol.1975.sp011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Fahim M. A. Progression of age changes in mature mouse motor nerve terminals and its relation to locomotor activity. J Neurocytol. 1985 Dec;14(6):1019–1036. doi: 10.1007/BF01224810. [DOI] [PubMed] [Google Scholar]
- Smith D. O. Acetylcholine storage, release and leakage at the neuromuscular junction of mature adult and aged rats. J Physiol. 1984 Feb;347:161–176. doi: 10.1113/jphysiol.1984.sp015059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]


