Abstract
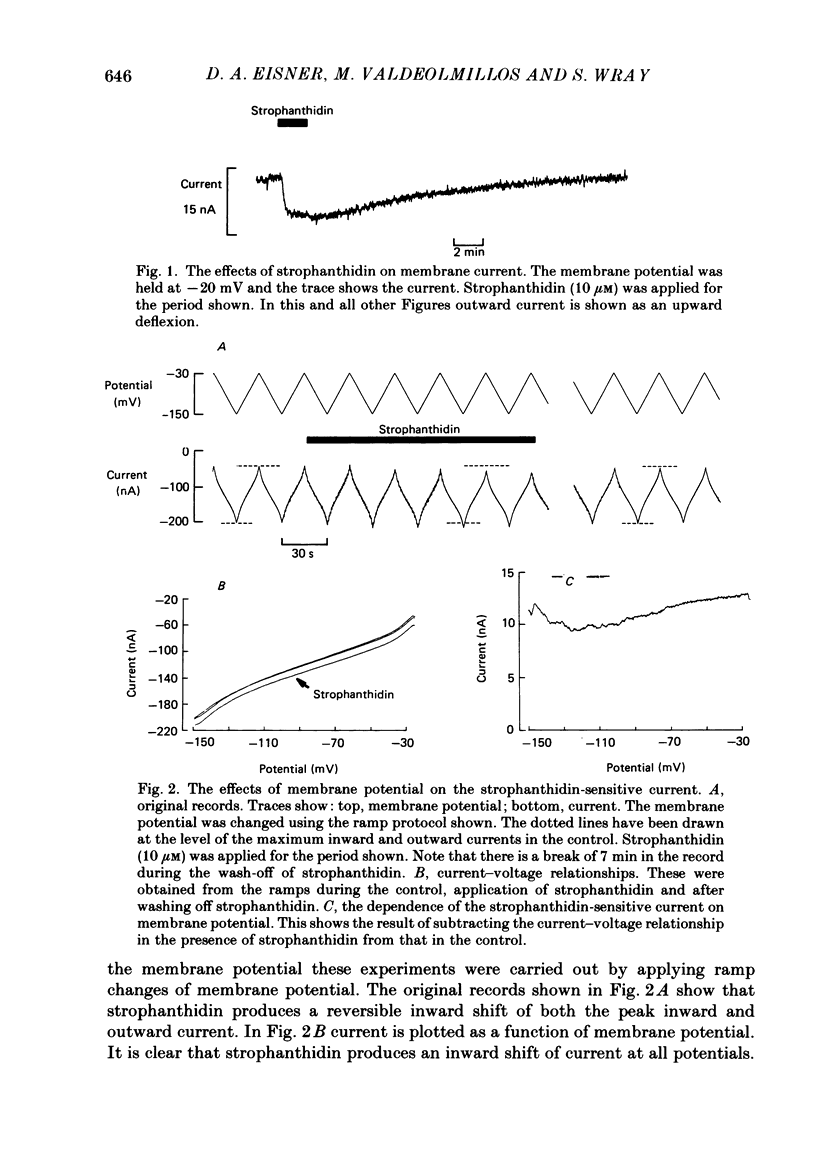
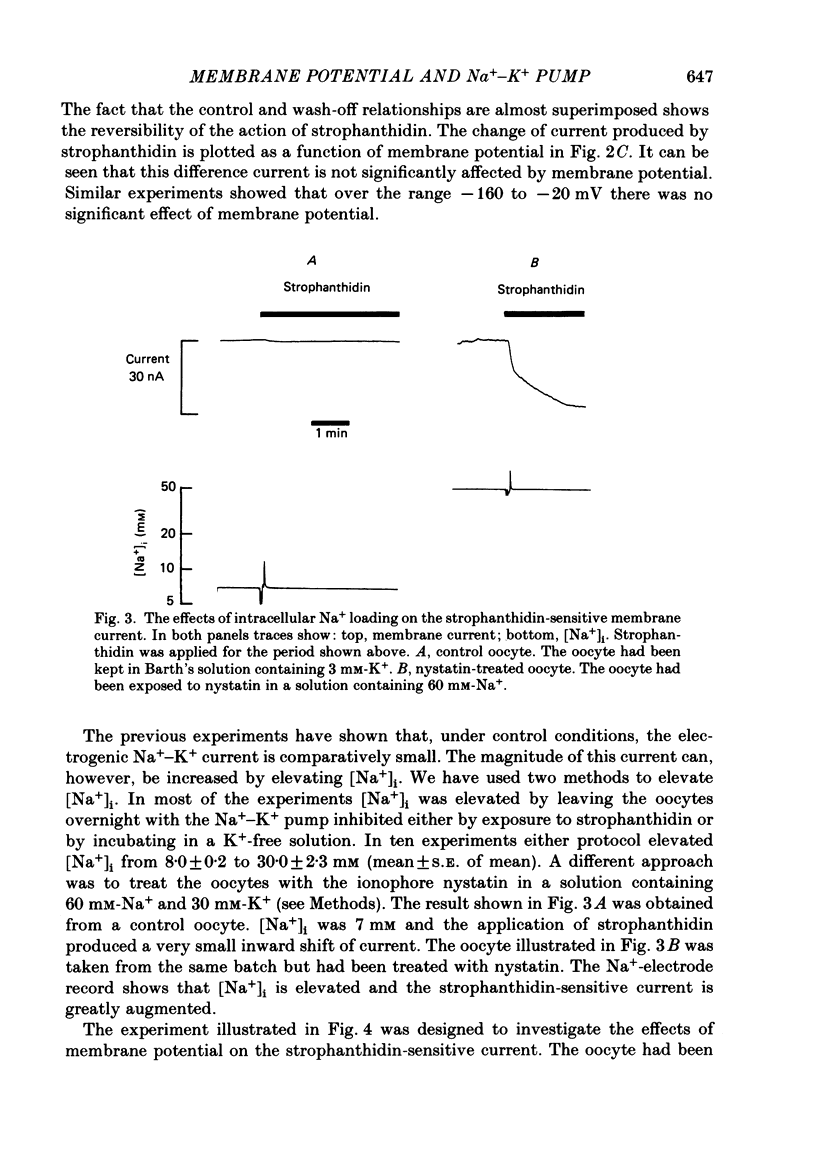
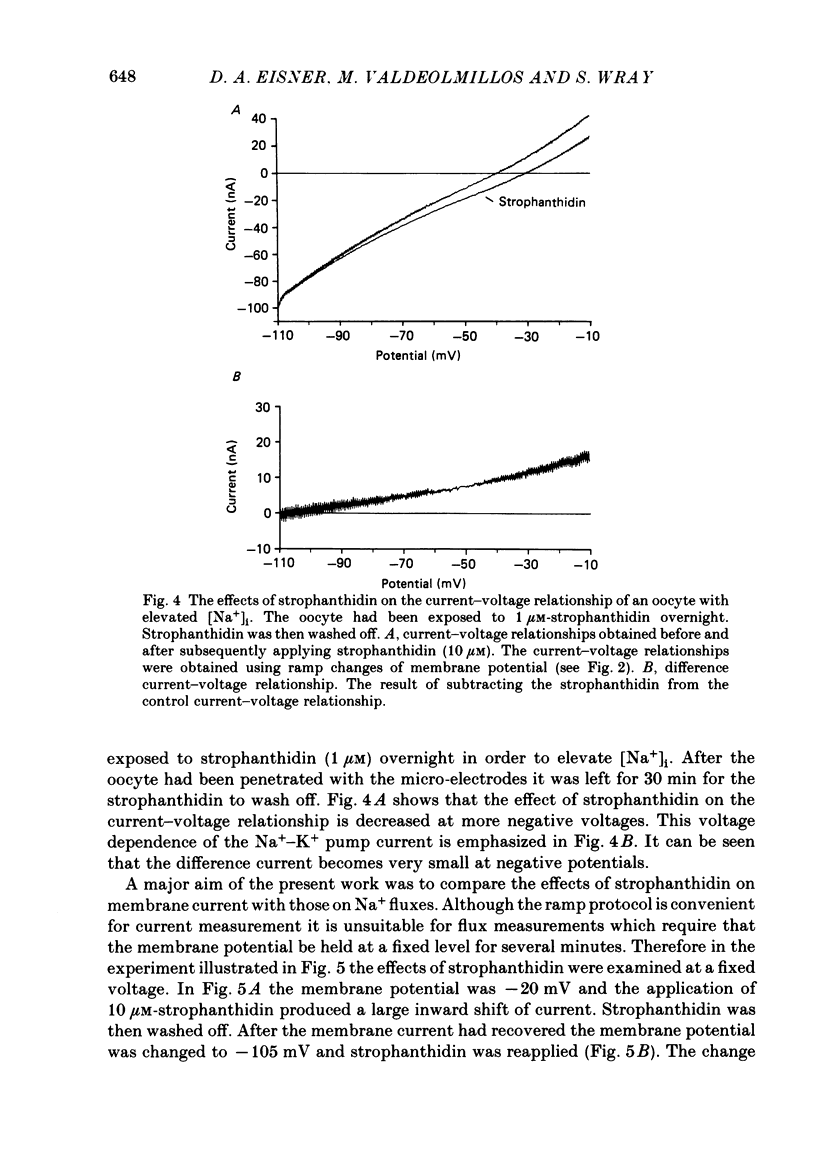
1. The effects of membrane potential on the Na+-K+ pump were studied by measuring membrane current and 22Na+ efflux in voltage-clamped Xenopus oocytes. The effects of inhibiting the Na+-K+ pump with strophanthidin were examined. 2. Strophanthidin produced an inward shift of membrane current which reversed on removal of the drug. In control oocytes the magnitude of this current was not significantly affected by changing membrane potential over the range -20 to -160 mV. 3. In another series of experiments the intracellular Na+ concentration ([Na+]i) was elevated either by overnight Na+-K+ pump inhibition (strophanthidin or exposure to K+-free solutions) or by loading with nystatin. This Na+-loading increased the magnitude of the strophanthidin-sensitive current. The ratio of strophanthidin-sensitive 22Na+ efflux:strophanthidin-sensitive current was consistent with that expected from a 3Na+-2K+ exchange. 4. When [Na+]i was elevated the strophanthidin-sensitive current was sensitive to changes of membrane potential. Hyperpolarization from -20 to -80 mV decreased the current to 60% of control. It is suggested that the current is not sensitive to membrane potential at normal [Na+]i because the over-all reaction is rate limited by the availability of intracellular Na+. 5. The application of strophanthidin decreased the rate of 22Na+ efflux. Both the strophanthidin-insensitive and the strophanthidin-sensitive components of efflux were sensitive to changes of membrane potential. The strophanthidin-insensitive component was not greatly affected by hyperpolarization from -40 to -160 mV but was increased by depolarization to +40 mV. 6. In Na+-loaded oocytes, the strophanthidin-sensitive component of 22Na+ efflux was inhibited by hyperpolarization negative from -40 mV. Hyperpolarization from -40 to -160 mV decreased the efflux by 54 +/- 5%. Over the limited range of potentials for which a comparison could be made, the effects on 22Na+ efflux were somewhat less than on the electrogenic Na+-K+ pump current. On average there was no significant effect of depolarizing from 0 to +40 mV. However, in some experiments a clear inhibition of the efflux was observed. If the oocytes were not Na+ loaded there was no significant effect of membrane potential on the strophanthidin-sensitive Na+ efflux. 7. These results show that the effects of membrane potential on the net reaction of the Na+-K+ pump (as measured by the electrogenic current) result partly from an inhibition of the forward mode of operation. However, there is also evidence to suggest a contribution from stimulation of the reverse reaction.
Full text
PDF


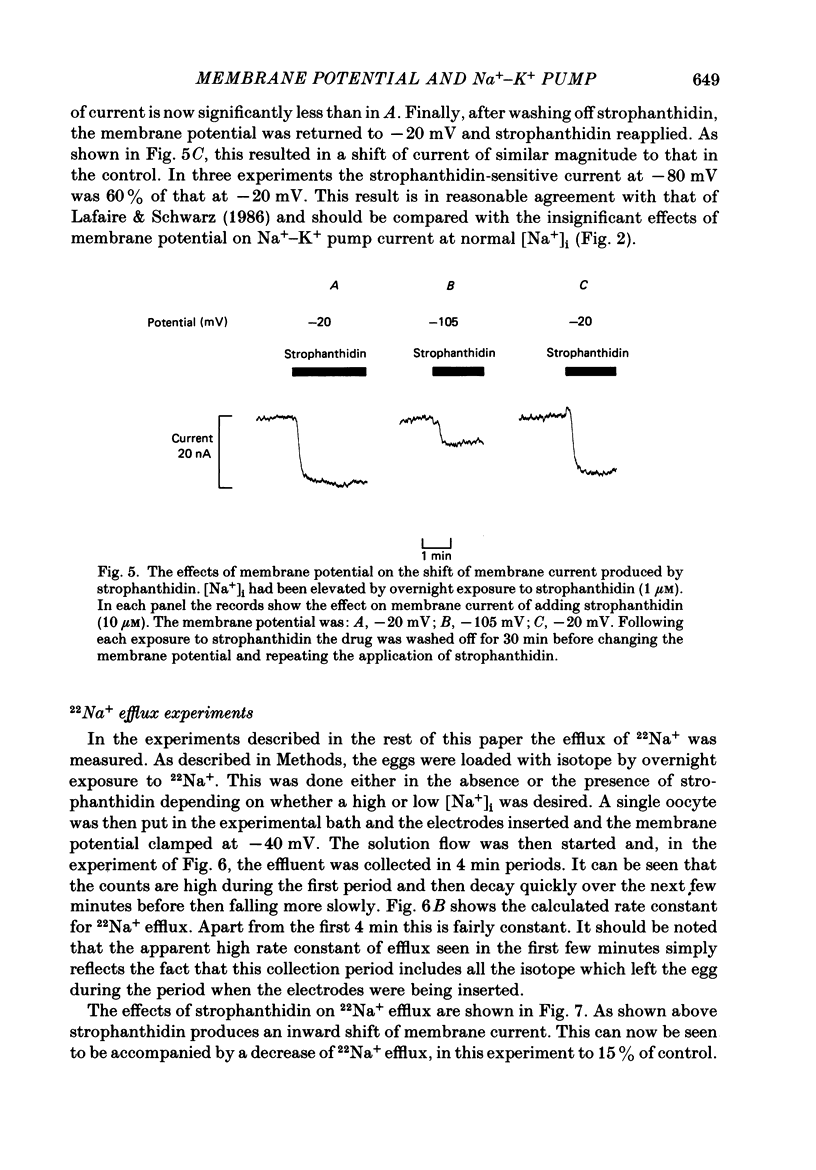






Selected References
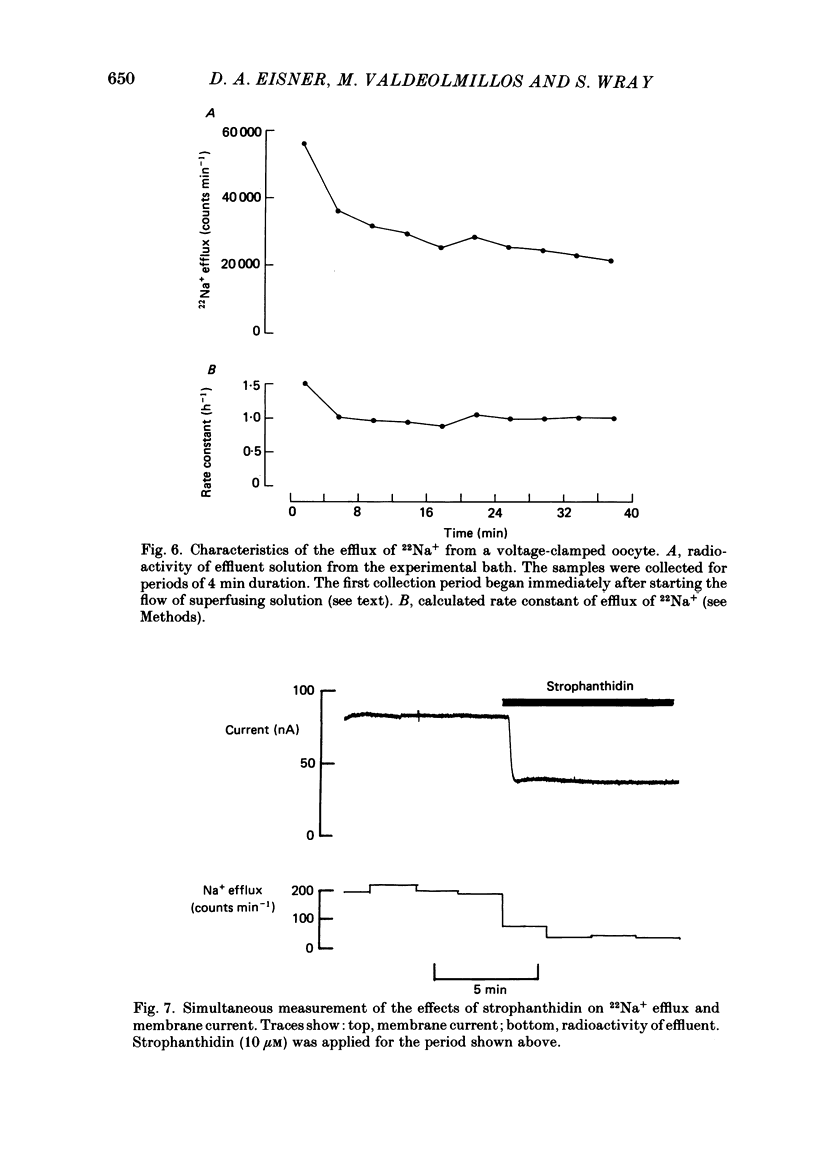
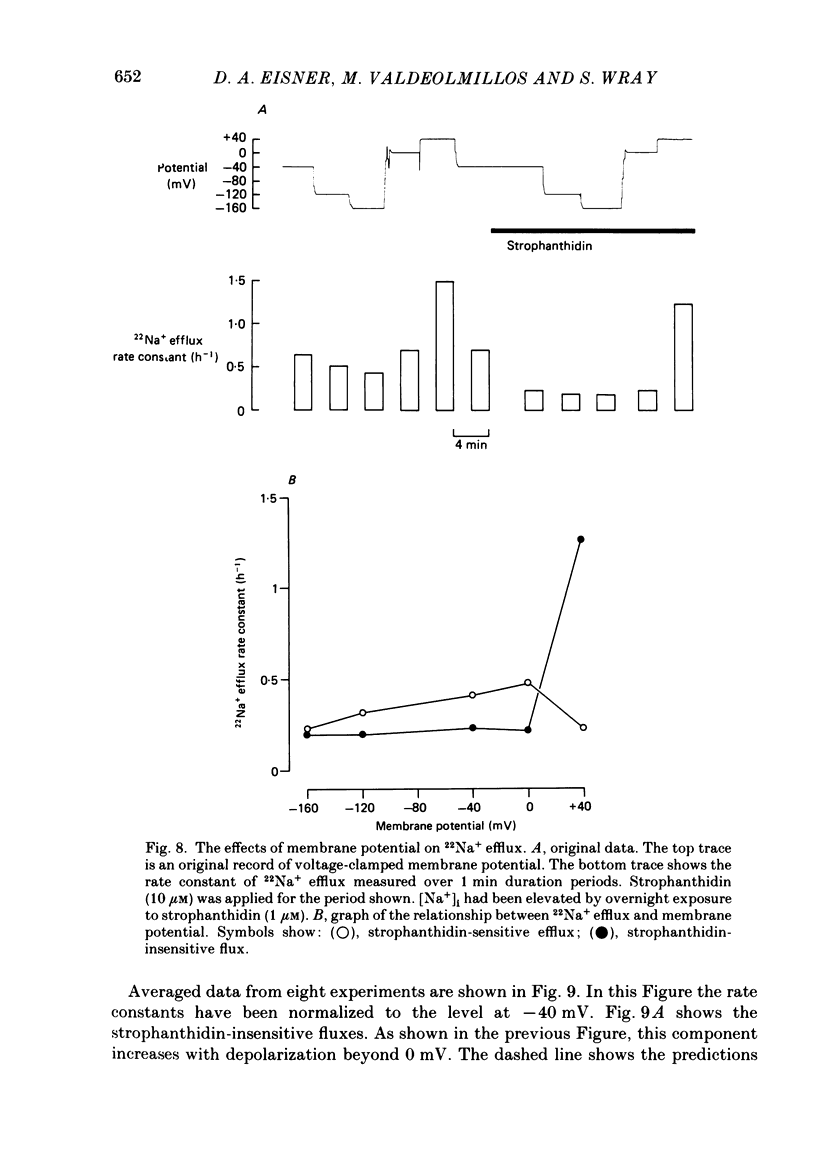
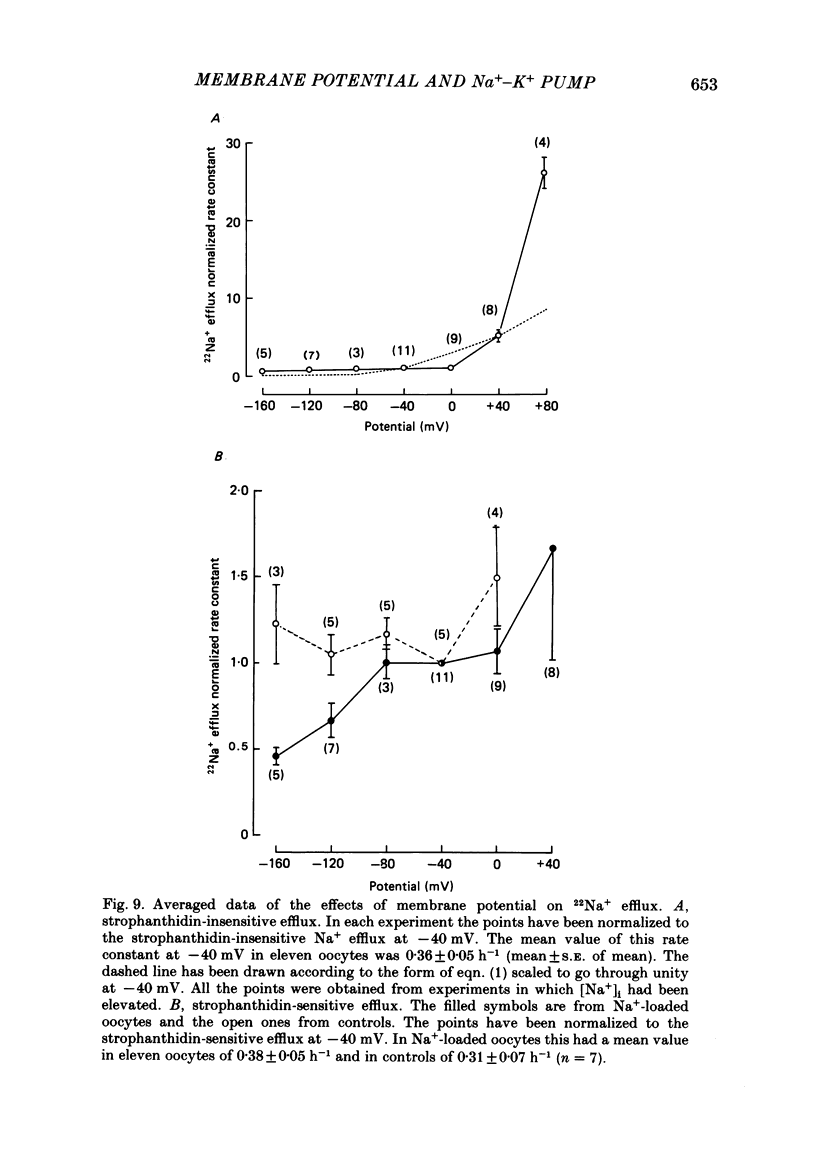
These references are in PubMed. This may not be the complete list of references from this article.
- Baud C., Kado R. T. Induction and disappearance of excitability in the oocyte of Xenopus laevis: a voltage-clamp study. J Physiol. 1984 Nov;356:275–289. doi: 10.1113/jphysiol.1984.sp015464. [DOI] [PMC free article] [PubMed] [Google Scholar]
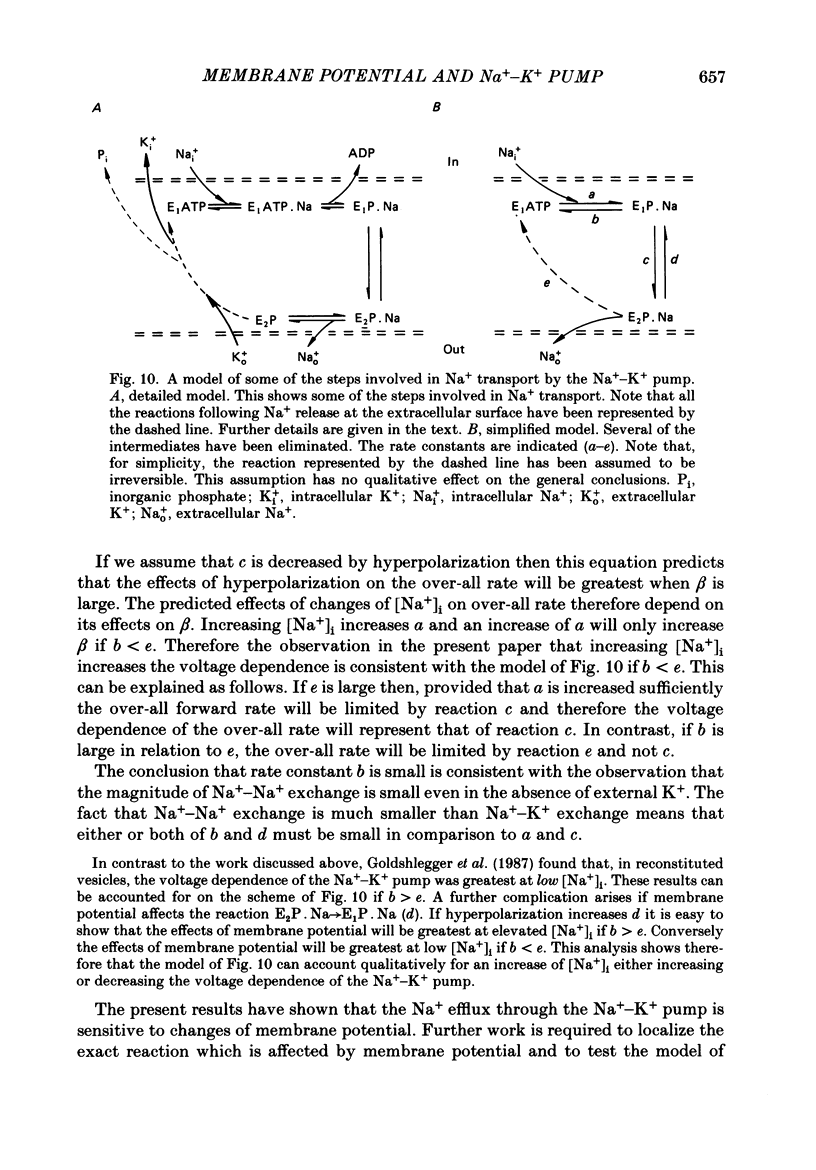
- Baud C., Kado R. T., Marcher K. Sodium channels induced by depolarization of the Xenopus laevis oocyte. Proc Natl Acad Sci U S A. 1982 May;79(10):3188–3192. doi: 10.1073/pnas.79.10.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Sjodin R. A. An analysis of the influence of membrane potential and metabolic poisoning with azide on the sodium pump in skeletal muscle. J Physiol. 1976 Dec;263(3):383–403. doi: 10.1113/jphysiol.1976.sp011636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Effects of membrane potential on sodium and potassium fluxes in squid axons. Ann N Y Acad Sci. 1974;242(0):406–433. doi: 10.1111/j.1749-6632.1974.tb19106.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Changes by acetylcholine of membrane currents in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:201–217. doi: 10.1113/jphysiol.1986.sp015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterrell D., Whittam R. The influence of the chloride gradient across red cell membranes on sodium and potassium movements. J Physiol. 1971 May;214(3):509–536. doi: 10.1113/jphysiol.1971.sp009446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICK D. A., LEA E. J. NA FLUXES IN SINGLE TOAD OOCYTES WITH SPECIAL REFERENCE TO THE EFFECT OF EXTERNAL AND INTERNAL NA CONCENTRATION ON NA EFFLUX. J Physiol. 1964 Oct;174:55–90. doi: 10.1113/jphysiol.1964.sp007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Rakowski R. F. Current generated by backward-running electrogenic Na pump in squid giant axons. 1984 May 31-Jun 6Nature. 309(5967):450–452. doi: 10.1038/309450a0. [DOI] [PubMed] [Google Scholar]
- Dick D. A., Fry D. J. Sodium fluxes in single amphibian oocytes: further studies and a new model. J Physiol. 1975 May;247(1):91–116. doi: 10.1113/jphysiol.1975.sp010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H., Schumacher T., Verdonck F. An identification of the K activated Na pump current in sheep Purkinje fibres. Pflugers Arch. 1982 Sep;394(3):256–263. doi: 10.1007/BF00589101. [DOI] [PubMed] [Google Scholar]
- Glynn I. M. The electrogenic sodium pump. Soc Gen Physiol Ser. 1984;38:33–48. [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Lafaire A. V., Schwarz W. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J Membr Biol. 1986;91(1):43–51. doi: 10.1007/BF01870213. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nelson M. T. Sodium pump stoicheiometry determined by simultaneous measurements of sodium efflux and membrane current in barnacle. J Physiol. 1984 Mar;348:665–677. doi: 10.1113/jphysiol.1984.sp015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L. Electrogenic sodium pumping in Xenopus blastomeres: apparent pump conductance and reversal potential. Soc Gen Physiol Ser. 1984;38:345–351. [PubMed] [Google Scholar]
- Zade-Oppen A. M., Schooler J. M., Jr, Cook P., Tosteson D. C. Effect of membrane potential and internal pH on active sodium-potassium transport and on ATP content in high-potassium sheep erythrocytes. Biochim Biophys Acta. 1979 Aug 7;555(2):285–298. doi: 10.1016/0005-2736(79)90168-8. [DOI] [PubMed] [Google Scholar]


