Abstract
1. Single fibres isolated from the tibialis anterior muscle of Rana temporaria were stimulated to produce a 1 s fused tetanus, while a short (ca. 0.5 mm) segment of the fibre was held at constant length. The segments were defined by opaque markers of hair that were placed on the fibre surface. The distance between two adjacent markers (one segment) was monitored by means of a photo-electric recording system. The length of a given segment could be controlled to within 0.2% of the segment's length by adjusting the over-all length of the fibre by means of an electromagnetic puller and servo system. 2. Segments producing constant force (no 'tension creep') during length-clamp recording were studied at different striation spacings within the following ranges of sarcomere length: 2.20-3.70, 1.90-2.45 and 1.50-2.20 microns. The absence of tension creep suggested (Edman & Reggiani, 1984 a) that the sarcomere pattern remained stable within the length-clamped segment during contraction at different lengths. 3. The tetanic force of a given length-clamped segment was consistently found to increase, as the sarcomere length was reduced from 2.20 to 1.98-2.02 microns, the mean increase in force being 6.9 +/- 0.4% (S.E. of mean, thirty-two segments). By further decreasing the sarcomere length active force was reduced. 4. The increase in force-producing capability between 2.20 and 2.00 microns sarcomere length was further explored by recording the maximum rate of force redevelopment, dF/dtmax, after a quick release during the plateau of a fixed-end tetanus. dF/dtmax varied with sarcomere length between 2.20 and 2.00 microns in the same way as the isometric force of a short, length-clamped segment, increasing by ca. 10% over this range. This finding provides further support for the view that the fibre's capacity to produce force is not constant between 2.20 and 2.00 microns sarcomere length. 5. The descending limb of the length-tension relation extended between 2.00 and approximately 3.65 microns sarcomere length. Its middle, straight portion (between 2.30 and 3.30 microns sarcomere spacings) extrapolated to zero tension at 3.49 microns sarcomere length. The upper and lower portions of the descending limb were slightly curved (at sarcomere lengths less than 2.30 and greater than 3.30 microns, respectively) giving the descending limb a symmetrical sigmoid appearance.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





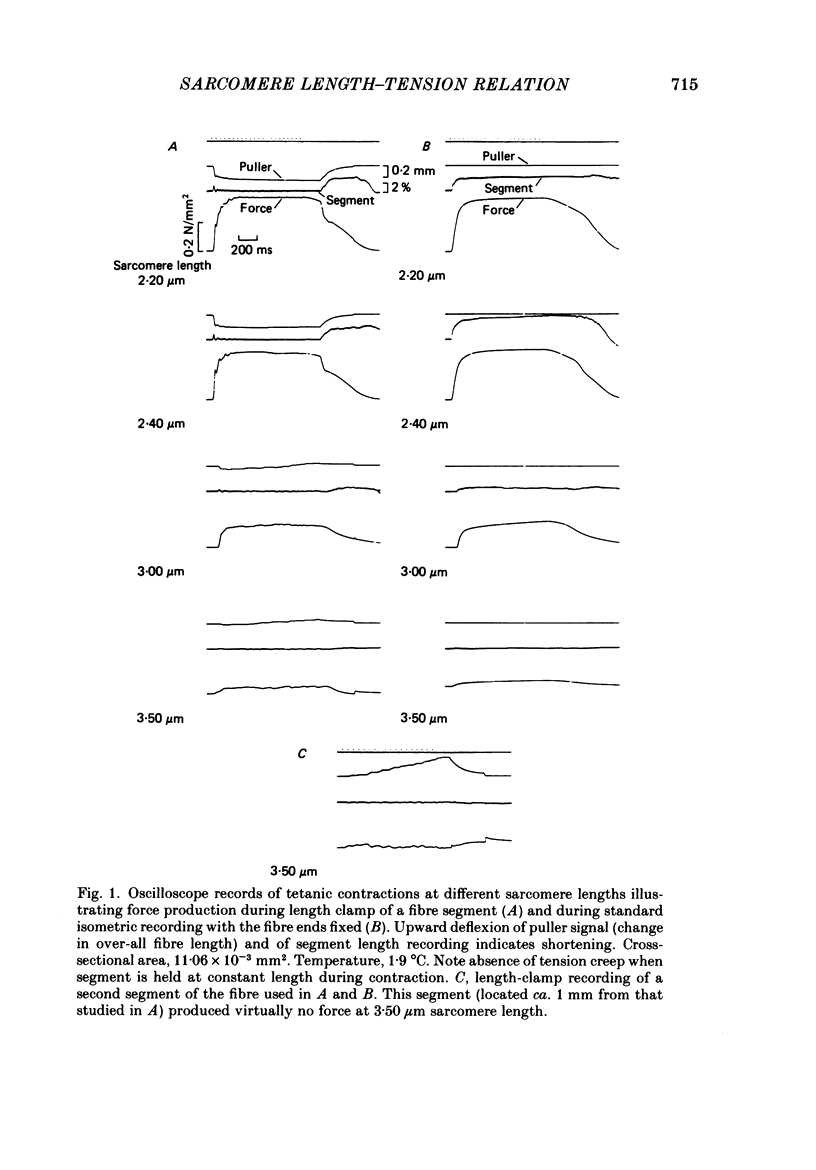
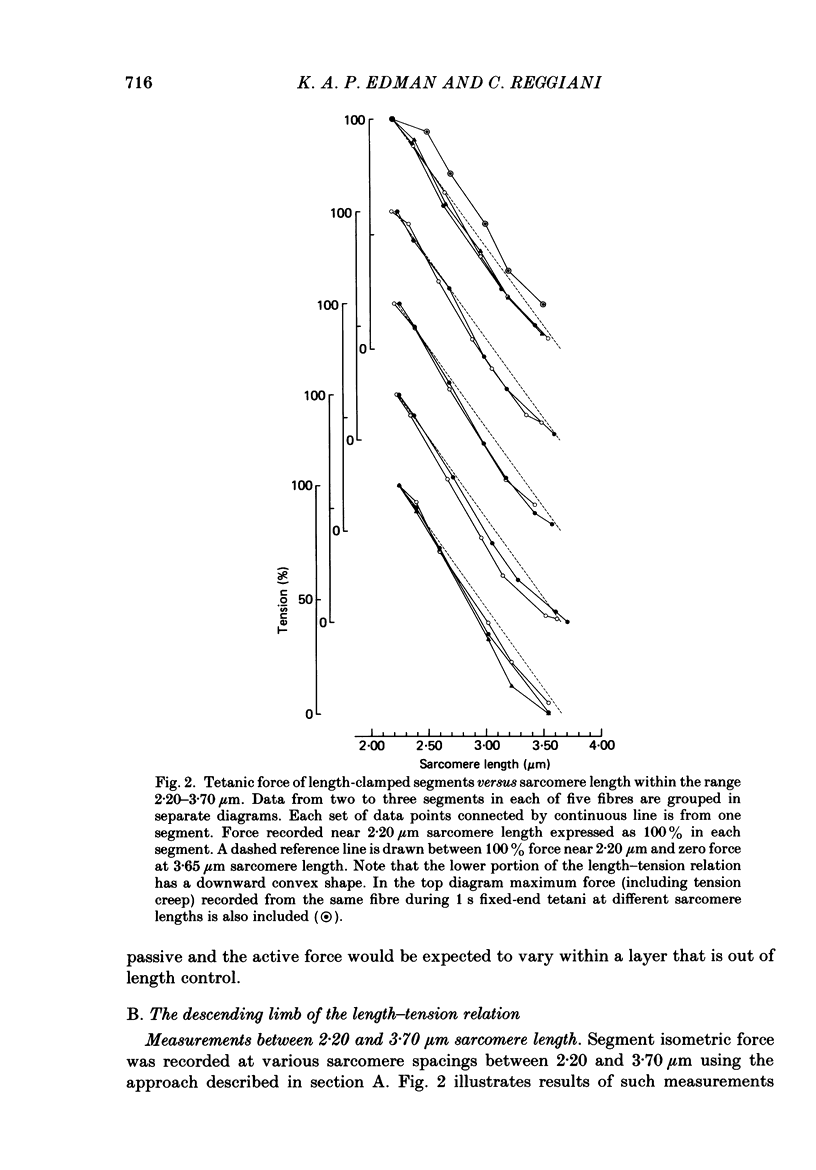
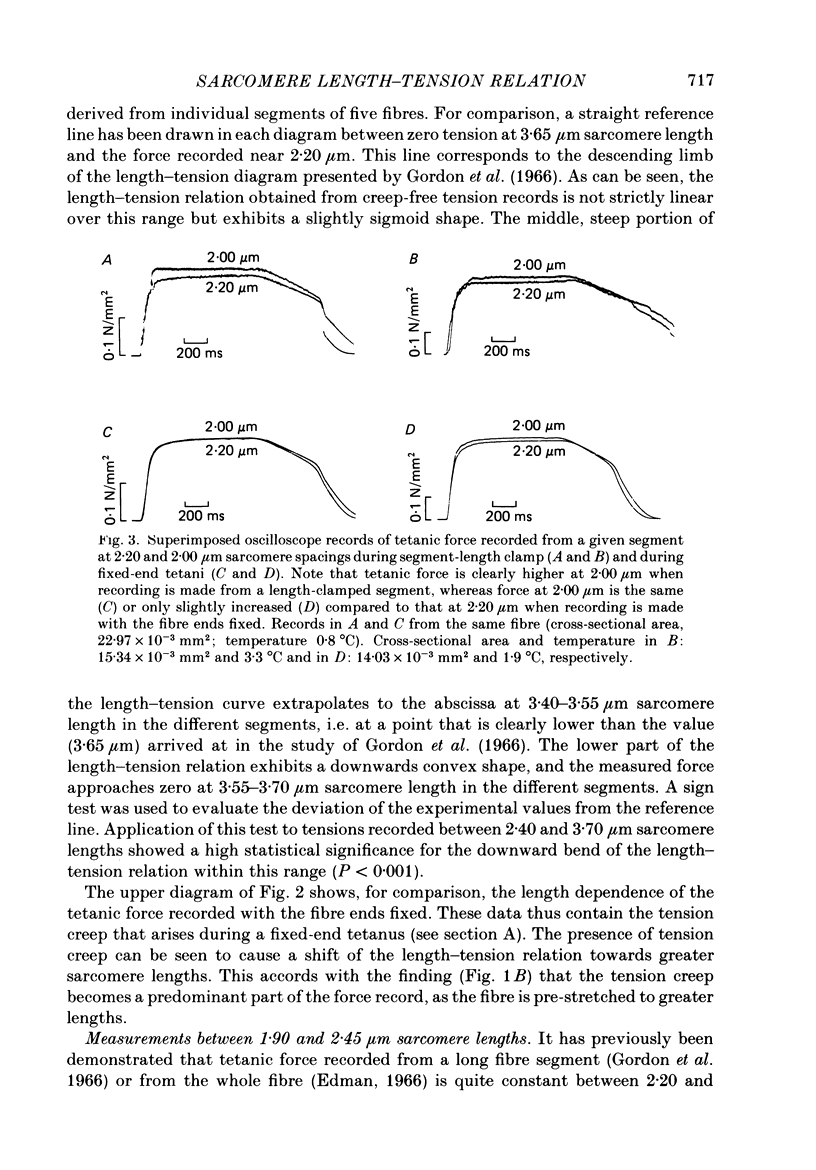
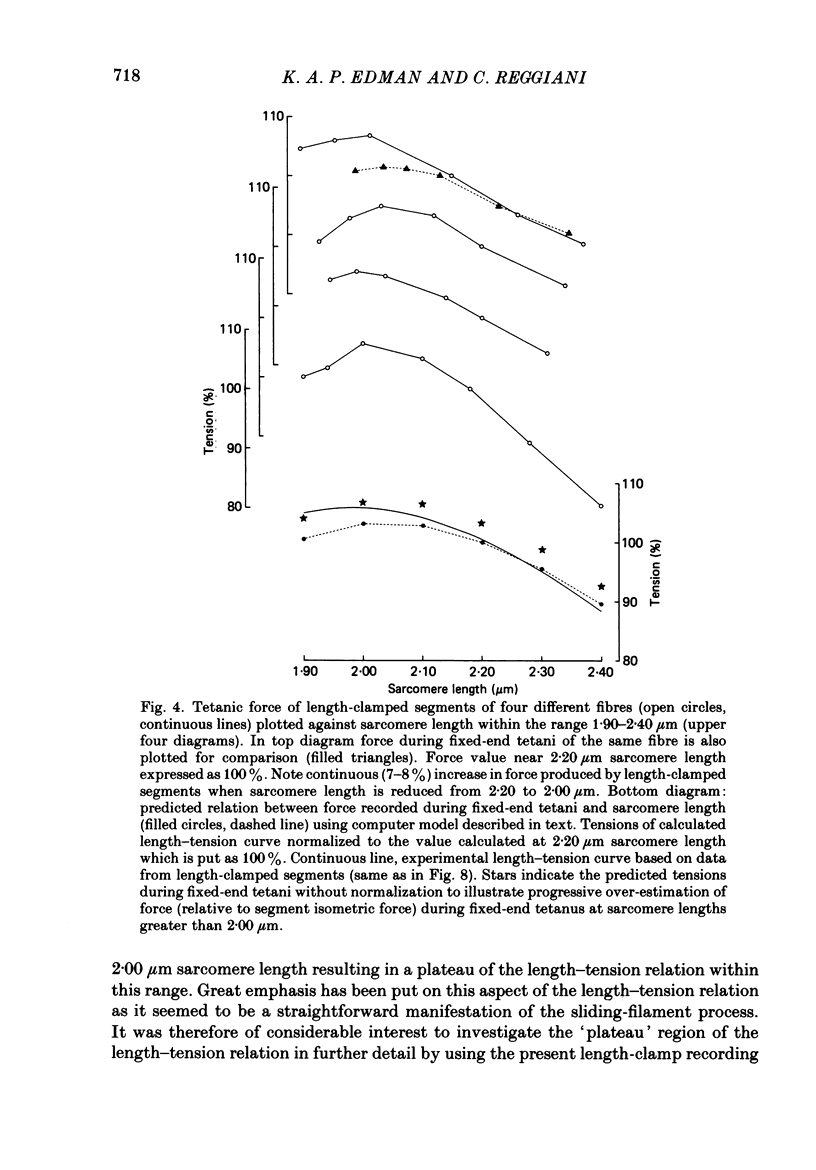

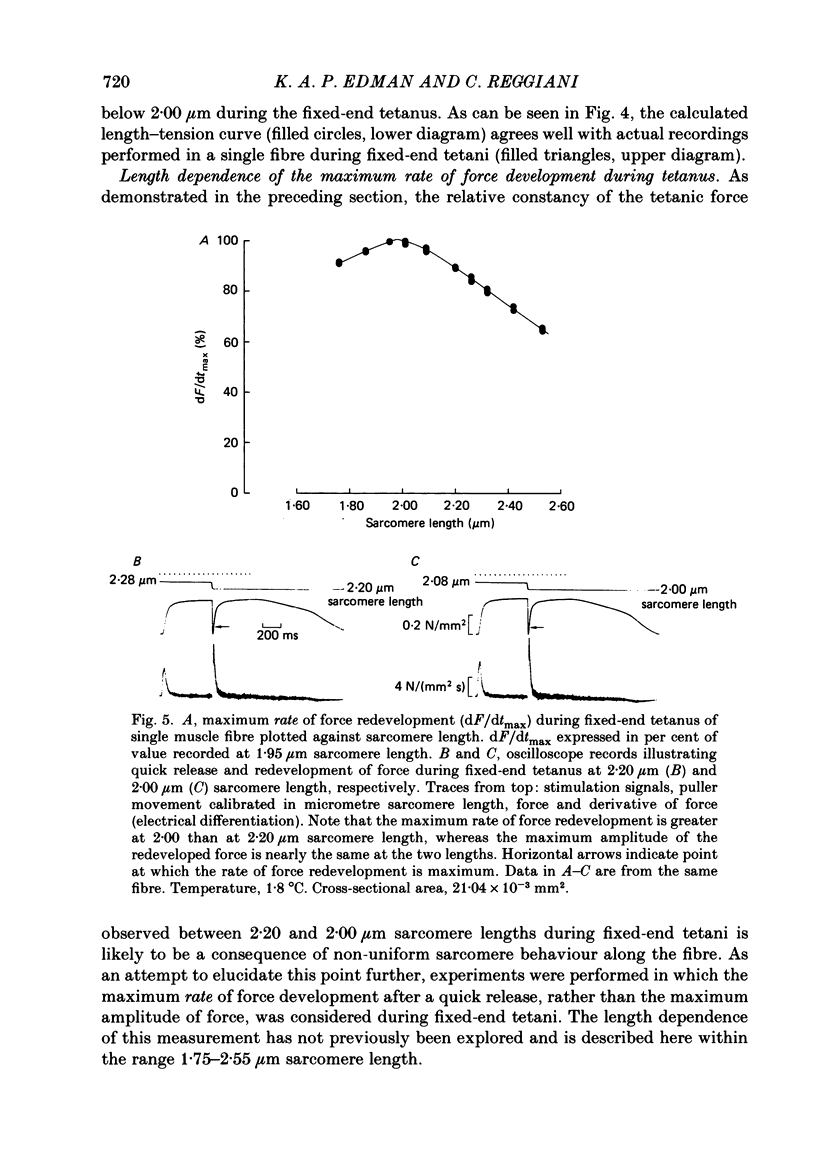

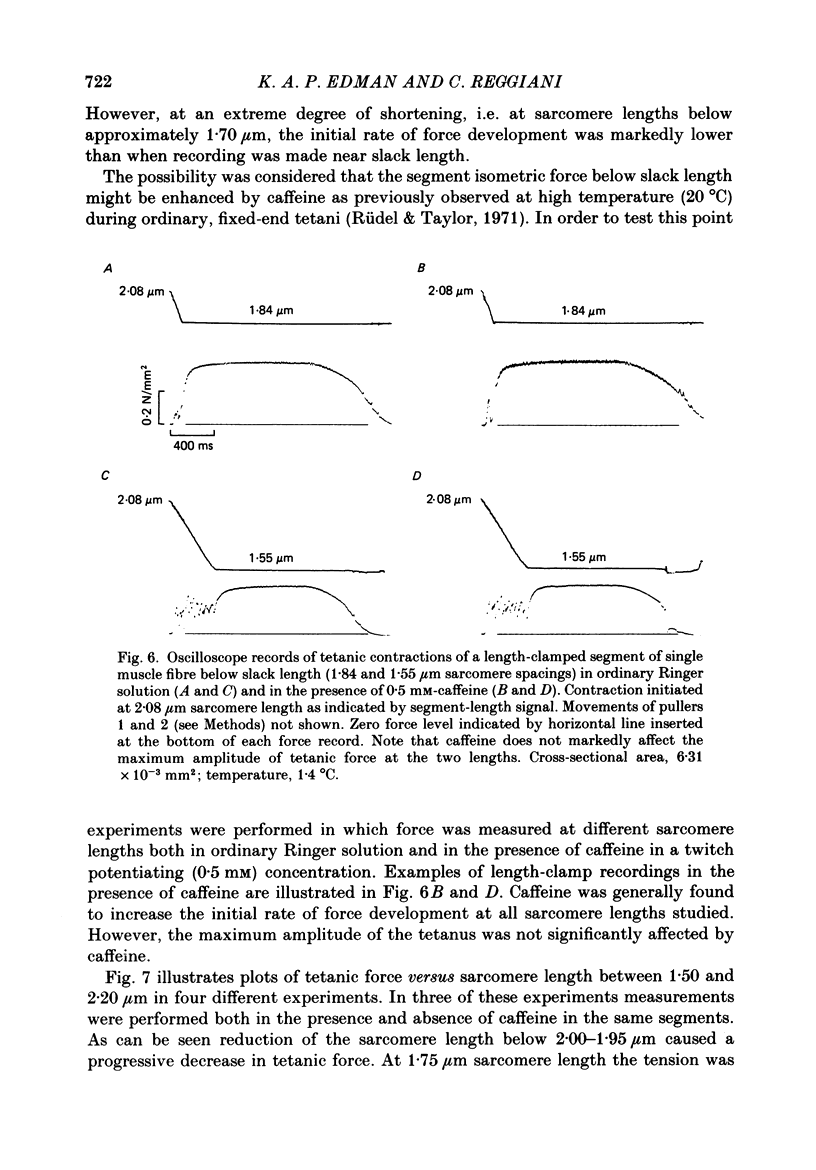
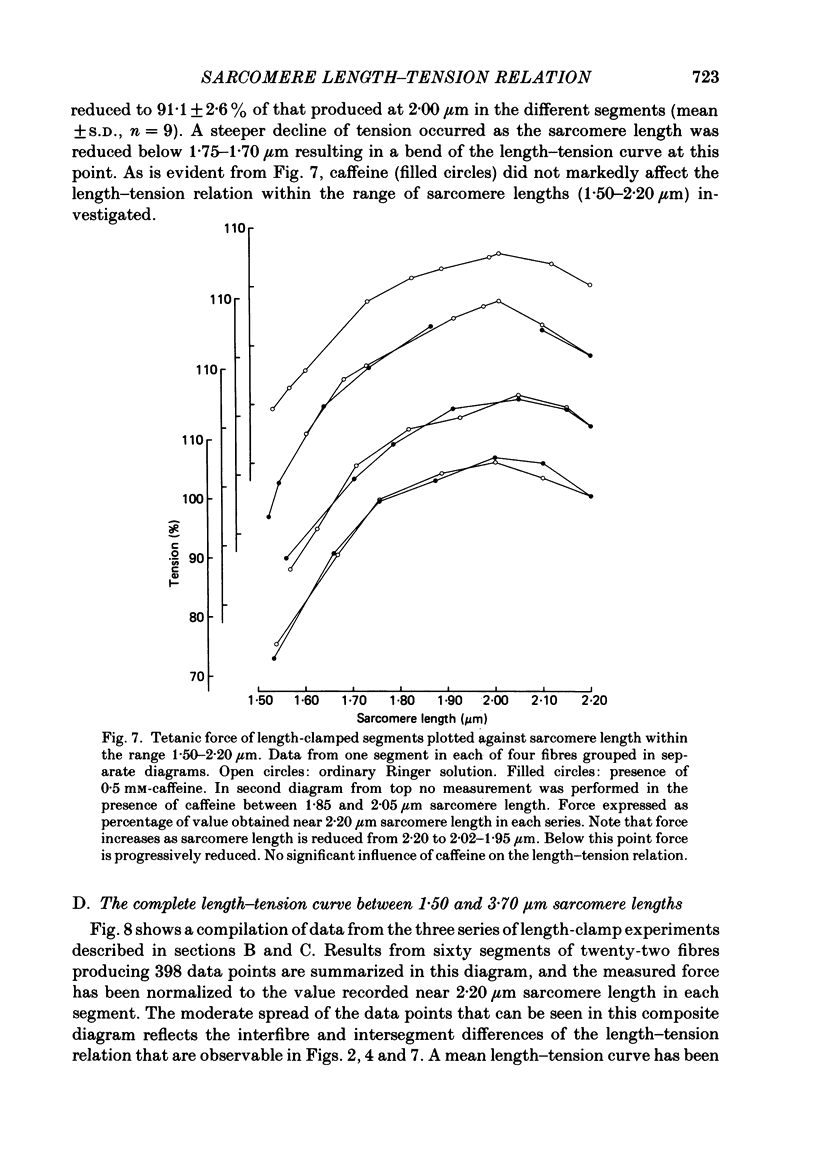
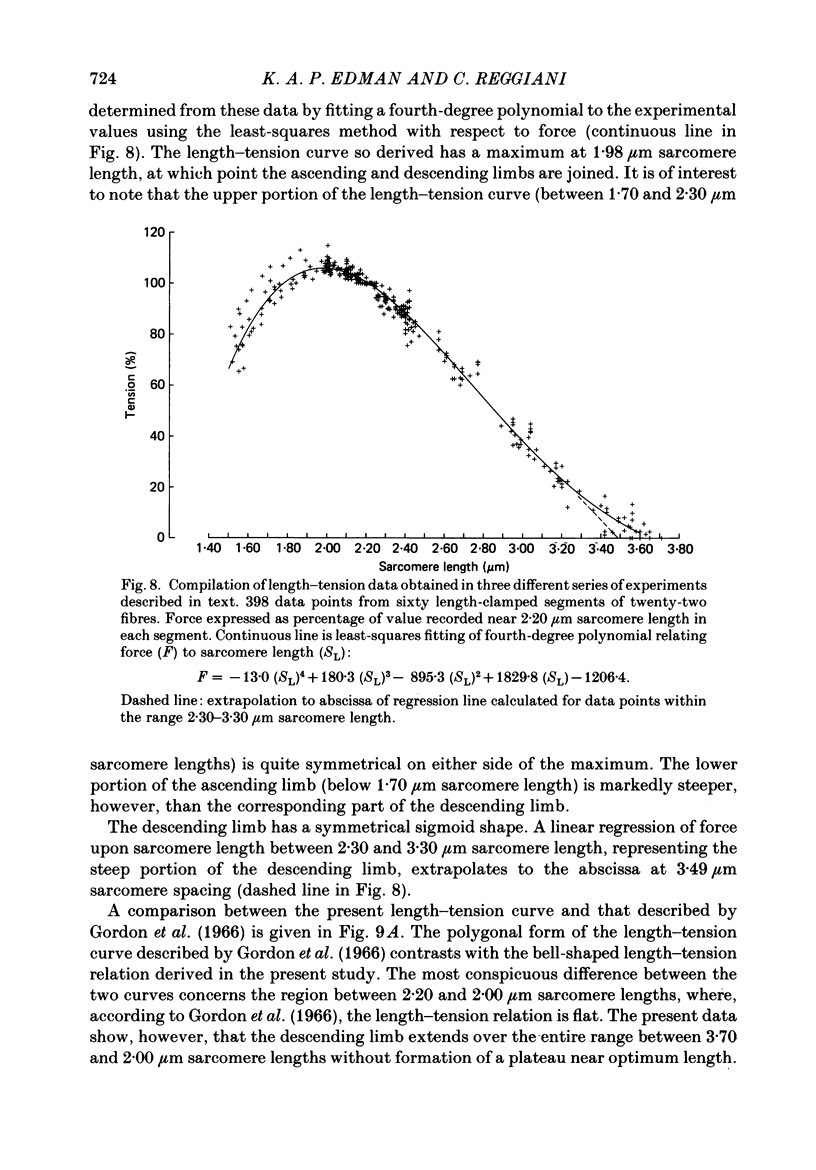







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Bottinelli R. The descending limb of the sarcomere length-force relation in single muscle fibres of the frog. J Muscle Res Cell Motil. 1985 Oct;6(5):585–600. doi: 10.1007/BF00711916. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A., Sherratt R. M. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol. 1983 Aug;341:41–58. doi: 10.1113/jphysiol.1983.sp014791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. M., Hill L. Mercuric chloride in alcohol and chloroform used as a rapidly acting fixative for contracting muscle fibres. J Microsc. 1982 Mar;125(Pt 3):319–336. doi: 10.1111/j.1365-2818.1982.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Offer G. Axial arrangement of crossbridges in thick filaments of vertebrate skeletal muscle. J Mol Biol. 1976 Apr 5;102(2):325–332. doi: 10.1016/s0022-2836(76)80057-5. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Andersson K. E. The variation in active tension with sarcomere length in vertebrate skeletal muscle and its relation to fibre width. Experientia. 1968 Feb 15;24(2):134–136. doi: 10.1007/BF02146942. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Absence of plateau of the sarcomere length-tension relation in frog muscle fibres. Acta Physiol Scand. 1984 Oct;122(2):213–216. doi: 10.1111/j.1748-1716.1984.tb07502.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J Physiol. 1966 Mar;183(2):407–417. doi: 10.1113/jphysiol.1966.sp007873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Intersarcomere dynamics during fixed-end tetanic contractions of frog muscle fibres. J Physiol. 1979 Aug;293:365–378. doi: 10.1113/jphysiol.1979.sp012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. Fine structure of tortoise skeletal muscle. J Physiol. 1968 Aug;197(3):709–715. doi: 10.1113/jphysiol.1968.sp008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R., Taylor S. R. Striated muscle fibers: facilitation of contraction at short lengths by caffeine. Science. 1971 Apr 23;172(3981):387–389. doi: 10.1126/science.172.3981.387. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Podolsky R. J. Length-force relation of calcium activated muscle fibers. Science. 1972 Apr 7;176(4030):52–54. doi: 10.1126/science.176.4030.52. [DOI] [PubMed] [Google Scholar]
- Sugi H., Ohta T., Tameyasu T. Development of the maximum isometric force at short sarcomere lengths in calcium-activated muscle myofibrils. Experientia. 1983 Feb 15;39(2):147–148. doi: 10.1007/BF01958866. [DOI] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R. Striated muscle fibers: inactivation of contraction induced by shortening. Science. 1970 Feb 6;167(3919):882–884. doi: 10.1126/science.167.3919.882. [DOI] [PubMed] [Google Scholar]
- Wang K. Cytoskeletal matrix in striated muscle: the role of titin, nebulin and intermediate filaments. Adv Exp Med Biol. 1984;170:285–305. doi: 10.1007/978-1-4684-4703-3_25. [DOI] [PubMed] [Google Scholar]
- ter Keurs H. E., Iwazumi T., Pollack G. H. The sarcomere length-tension relation in skeletal muscle. J Gen Physiol. 1978 Oct;72(4):565–592. doi: 10.1085/jgp.72.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]


