Abstract
1. Extracellular recordings were made from ninety-one neurones in the vicinity of the rostral trigeminal nucleus in chloralose-anaesthetized cats. 2. Sixty-two neurones within this area were activated by electrical stimulation of the ipsilateral superior laryngeal nerve (s.l.n.). Only two of the twenty-one neurones tested had an additional input from the contralateral s.l.n. 3. Fifty of these sixty-two neurones were also activated synaptically by light mechanical stimulation of the ipsilateral nasal cavity and in the eight neurones tested electrical stimulation of the ipsilateral nostril evoked activity. All these neurones exhibited characteristics of postsynaptic responses to s.l.n. and nasal stimulation, showing a variable latency to onset to either stimulus, summation and facilitation of more than one stimulus. 4. None of those neurones receiving an s.l.n. input, or those with convergent inputs from the s.l.n. and nose, could be affected by mechanical stimulation of any part of the face. 5. The activity of a further twenty-nine neurones was also recorded within this same general region. Sixteen responded to movement of the whiskers, five to touching the skin of the lower jaw, two to touching the skin of the upper jaw, three to touch around the eyebrows and three to touching other parts of the face. None of these neurones were activated by s.l.n. stimulation. 6. The location of seventeen of these neurones showing a convergent s.l.n. and nasal input was determined histologically. They were closely grouped together in a region 3.5-4.5 mm rostral to obex in and around the main trigeminal sensory nucleus, dorsolateral to the retrofacial nucleus corresponding to the parvocellular division of the alaminar spinal trigeminal nucleus. 7. The lack of somatosensory input to those neurones receiving a convergent input from the nose and s.l.n. is discussed in relation to previous studies describing somatosensory-visceral convergence to neurones within trigeminal nuclei.
Full text
PDF

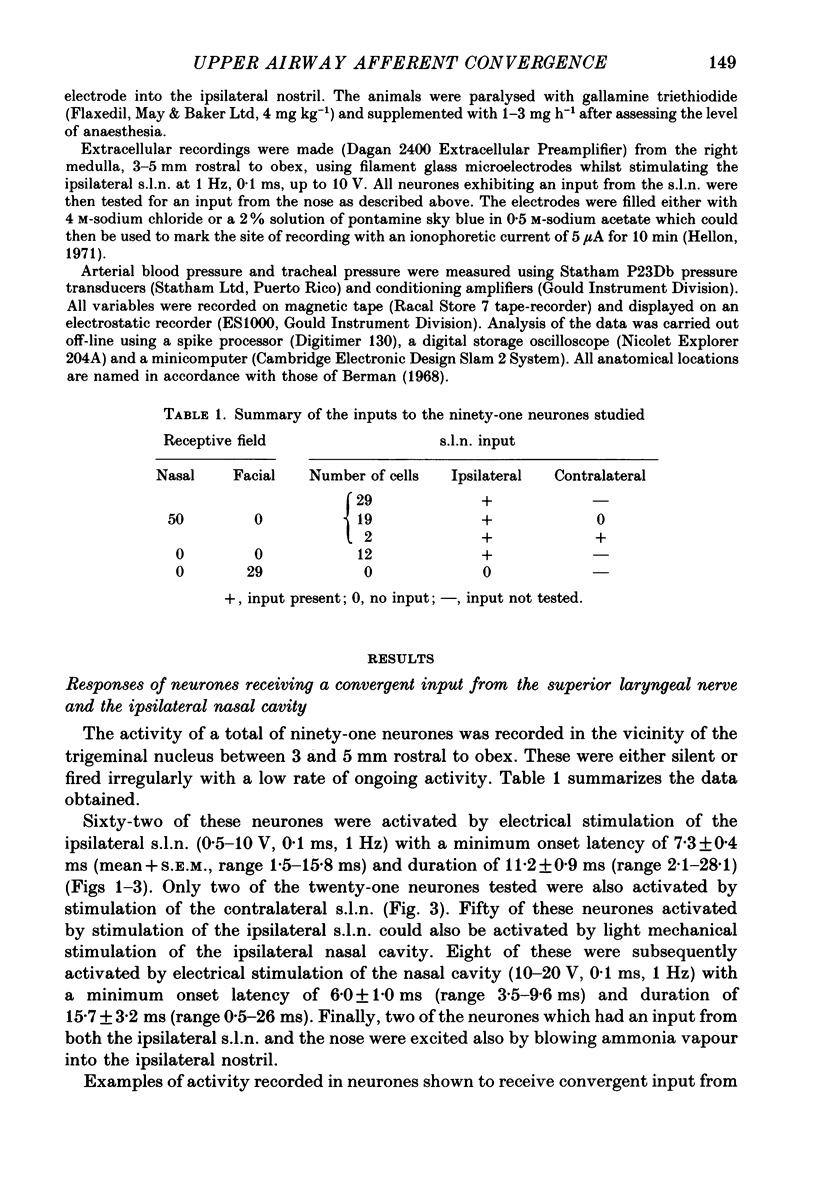
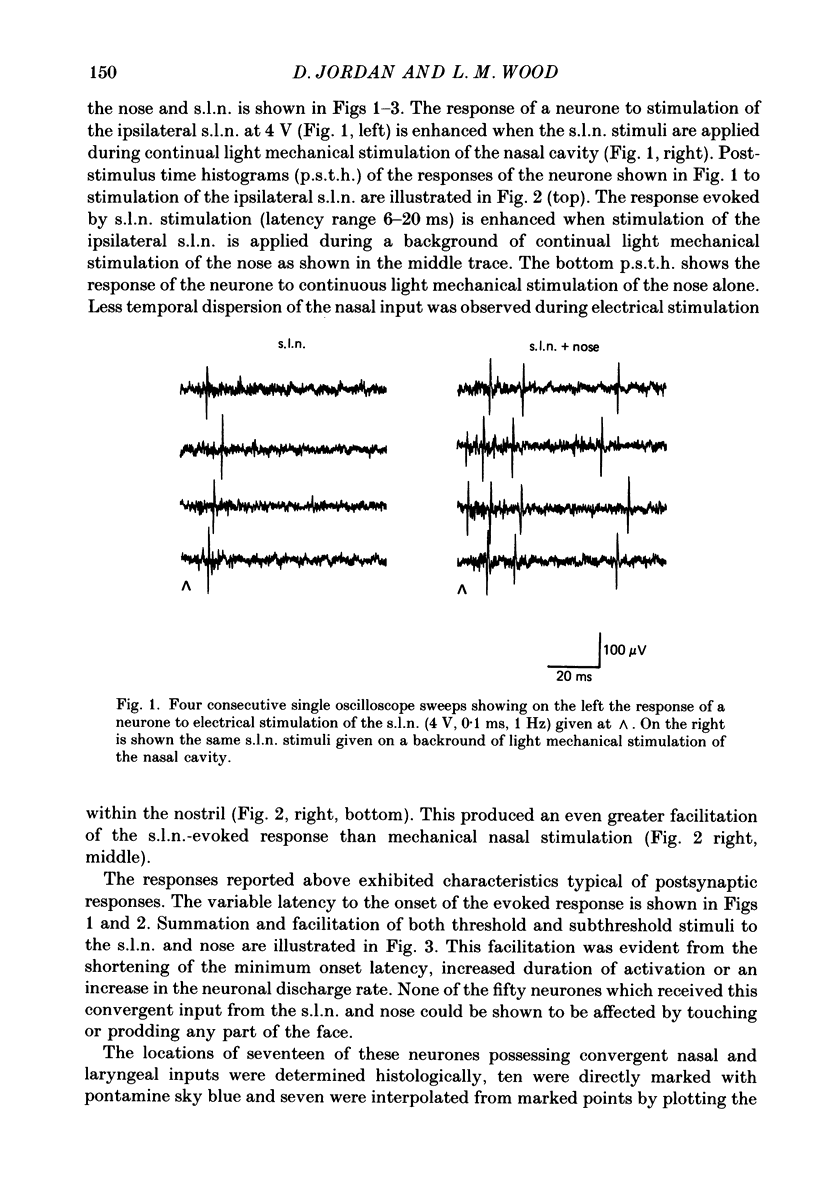
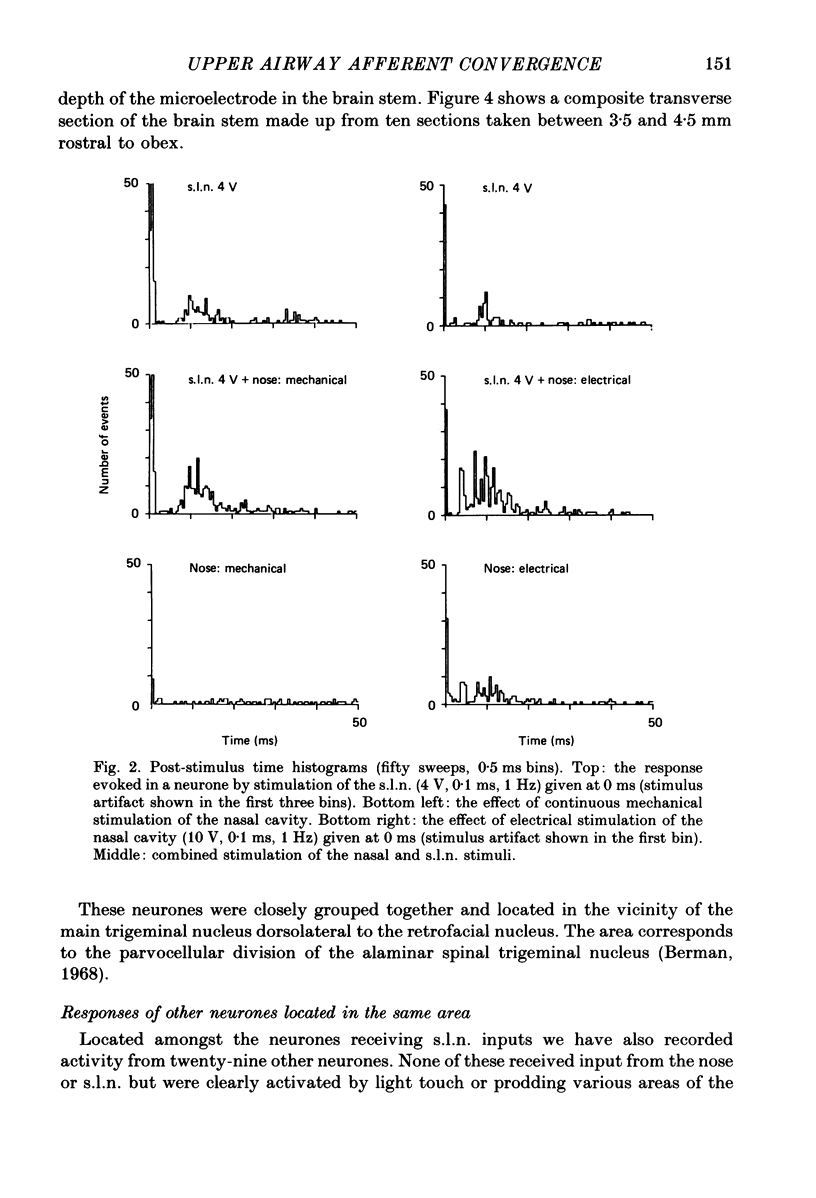
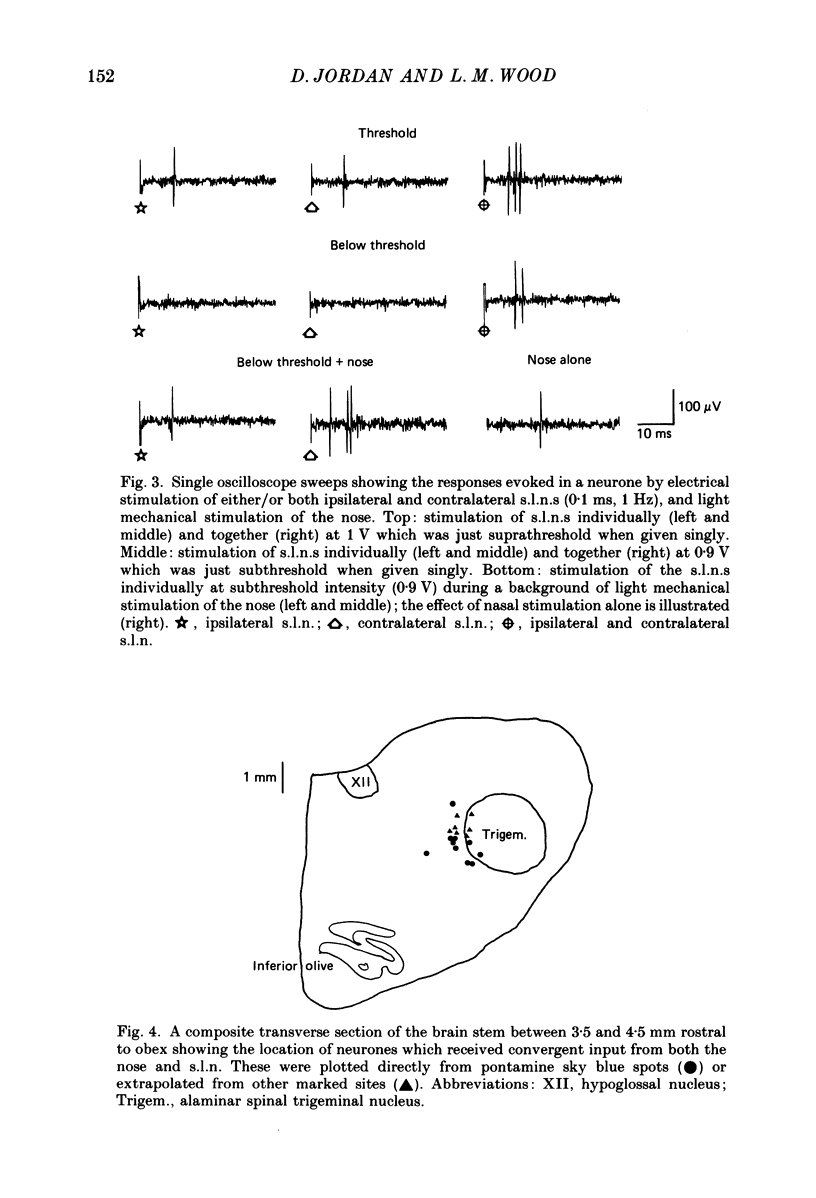



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angell-James J. E., Daly M. B. Some aspects of upper respiratory tract reflexes. Acta Otolaryngol. 1975 Mar-Apr;79(3-4):242–252. doi: 10.3109/00016487509124680. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Responses of cells in the brain stem of the cat to stimulation of the sinus, glossopharyngeal, aortic and superior laryngeal nerves. J Physiol. 1970 Aug;209(2):359–373. doi: 10.1113/jphysiol.1970.sp009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Car A., Jean A., Roman C. A pontine primary relay for ascending projections of the superior laryngeal nerve,. Exp Brain Res. 1975;22(2):197–210. doi: 10.1007/BF00237689. [DOI] [PubMed] [Google Scholar]
- DARIAN-SMITH I. Neurone activity in the cat's trigeminal main sensory nucleus elicited by graded afferent stimulation. J Physiol. 1960 Aug;153:52–73. doi: 10.1113/jphysiol.1960.sp006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G., RYAN R. D. FUNCTIONAL ORGANIZATION IN THE TRIGEMINAL MAIN SENSORY AND ROSTRAL SPINAL NUCLEI OF THE CAT. J Physiol. 1963 Aug;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K-M, Young D. W. Quantitative aspects of responses in trigeminal relay neurones and interneurones following mechanical stimulation of sinus hairs and skin in the cat. J Physiol. 1977 Oct;272(1):85–103. doi: 10.1113/jphysiol.1977.sp012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R. I., Hoye R. C., Ketcham A. S., Gould W. J. Hyposmia following laryngectomy. Lancet. 1968 Aug 31;2(7566):479–481. doi: 10.1016/s0140-6736(68)90647-8. [DOI] [PubMed] [Google Scholar]
- Henkin R. I., Larson A. L. On the mechanism of hyposmia following laryngectomy in man. Laryngoscope. 1972 May;82(5):836–843. doi: 10.1288/00005537-197205000-00010. [DOI] [PubMed] [Google Scholar]
- Hoye R. C., Ketcham A. S., Henkin R. I. Hyposmia after paranasal sinus exenteration of laryngectomy. Am J Surg. 1970 Oct;120(4):485–491. doi: 10.1016/s0002-9610(70)80012-5. [DOI] [PubMed] [Google Scholar]
- Hu J. W., Dostrovsky J. O., Sessle B. J. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J Neurophysiol. 1981 Feb;45(2):173–192. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- James J. E., De Burgh Daly M. Reflex respiratory and cardiovascular effects of stimulation of receptors in the nose of the dog. J Physiol. 1972 Feb;220(3):673–696. doi: 10.1113/jphysiol.1972.sp009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D., Paton J. F., Wood L. M. Effects of stimulation of nasal and superior laryngeal inputs on the hindlimb vasculature of anaesthetized cats. J Physiol. 1987 Dec;393:135–145. doi: 10.1113/jphysiol.1987.sp016815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR F. W. Facial, vagal and glossopharyngeal nerves in the cat. Afferent connections. Arch Neurol. 1962 Apr;6:264–281. doi: 10.1001/archneur.1962.00450220006003. [DOI] [PubMed] [Google Scholar]
- Moore-Gillon V. The nose after laryngectomy. J R Soc Med. 1985 Jun;78(6):435–439. doi: 10.1177/014107688507800603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle B. J. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Res. 1973 Apr 27;53(2):319–331. doi: 10.1016/0006-8993(73)90217-5. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Greenwood L. F. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: I. Responses to innocuous and noxious stimuli. Brain Res. 1976 Nov 26;117(2):211–226. doi: 10.1016/0006-8993(76)90731-9. [DOI] [PubMed] [Google Scholar]


