Abstract
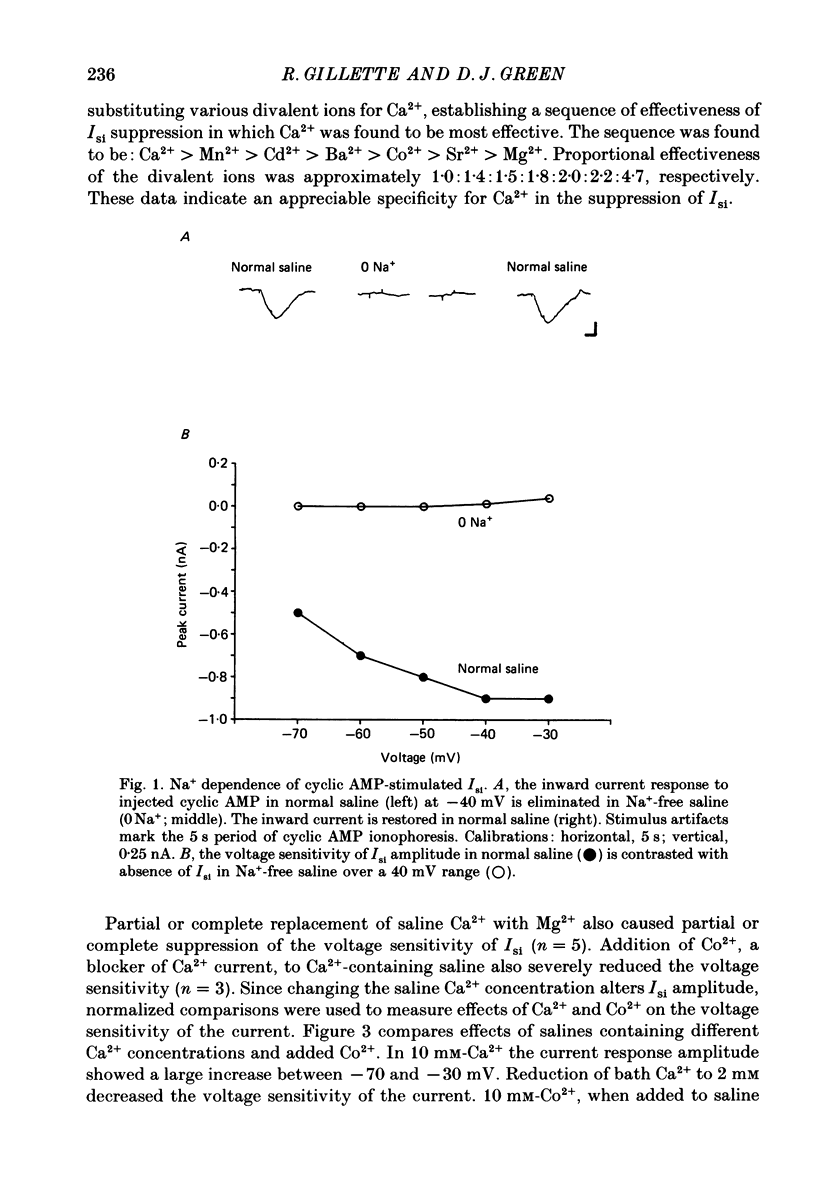
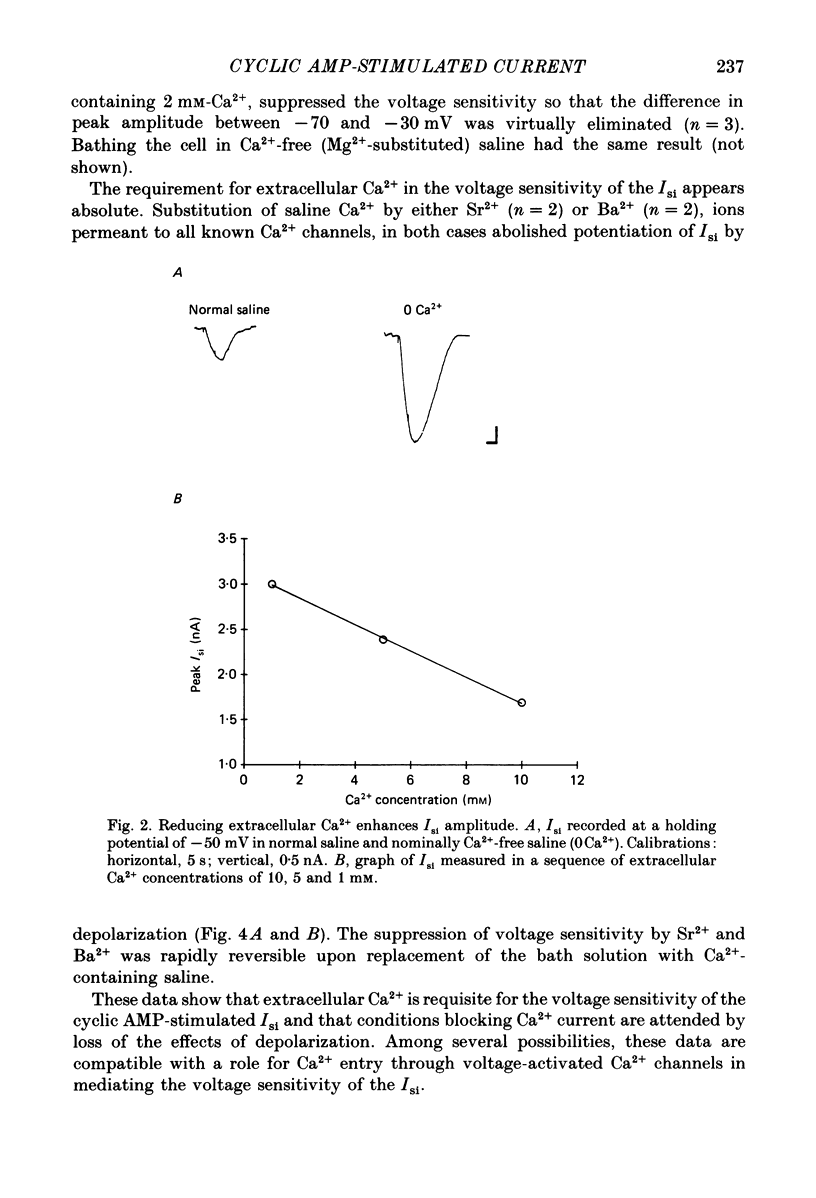
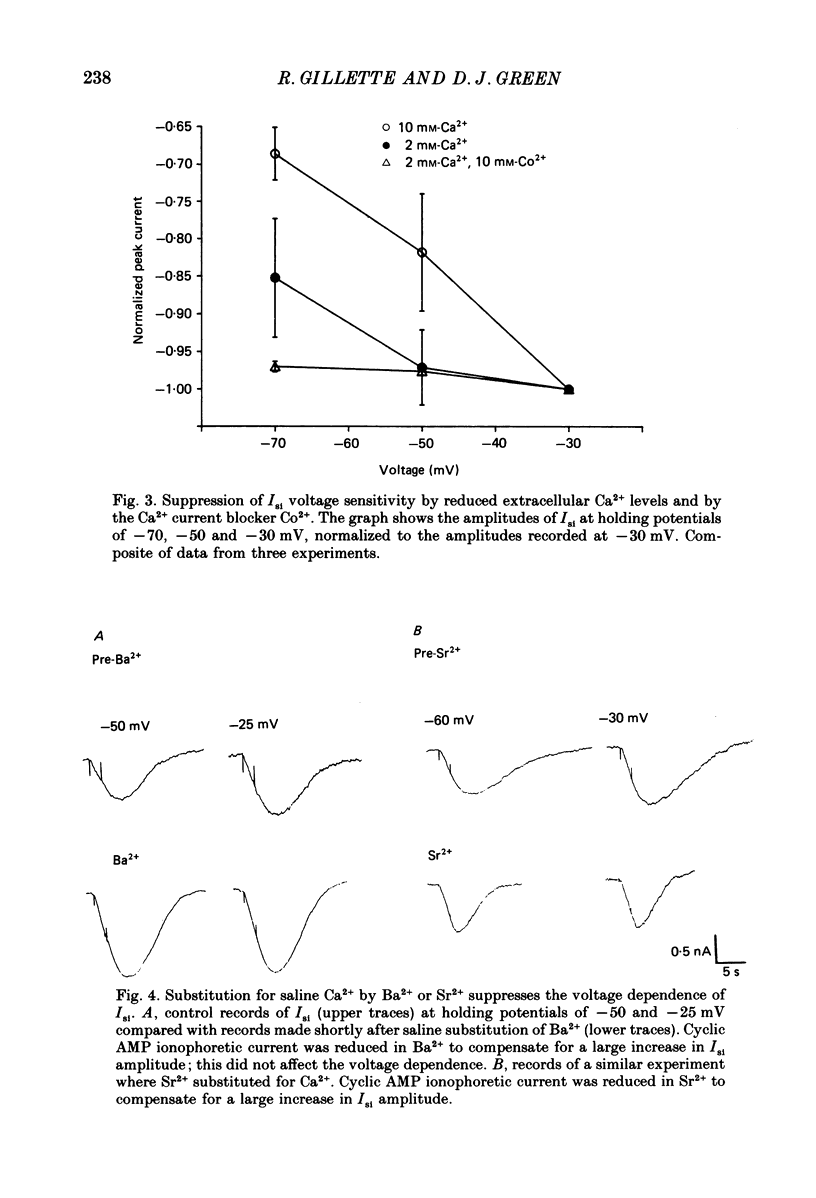
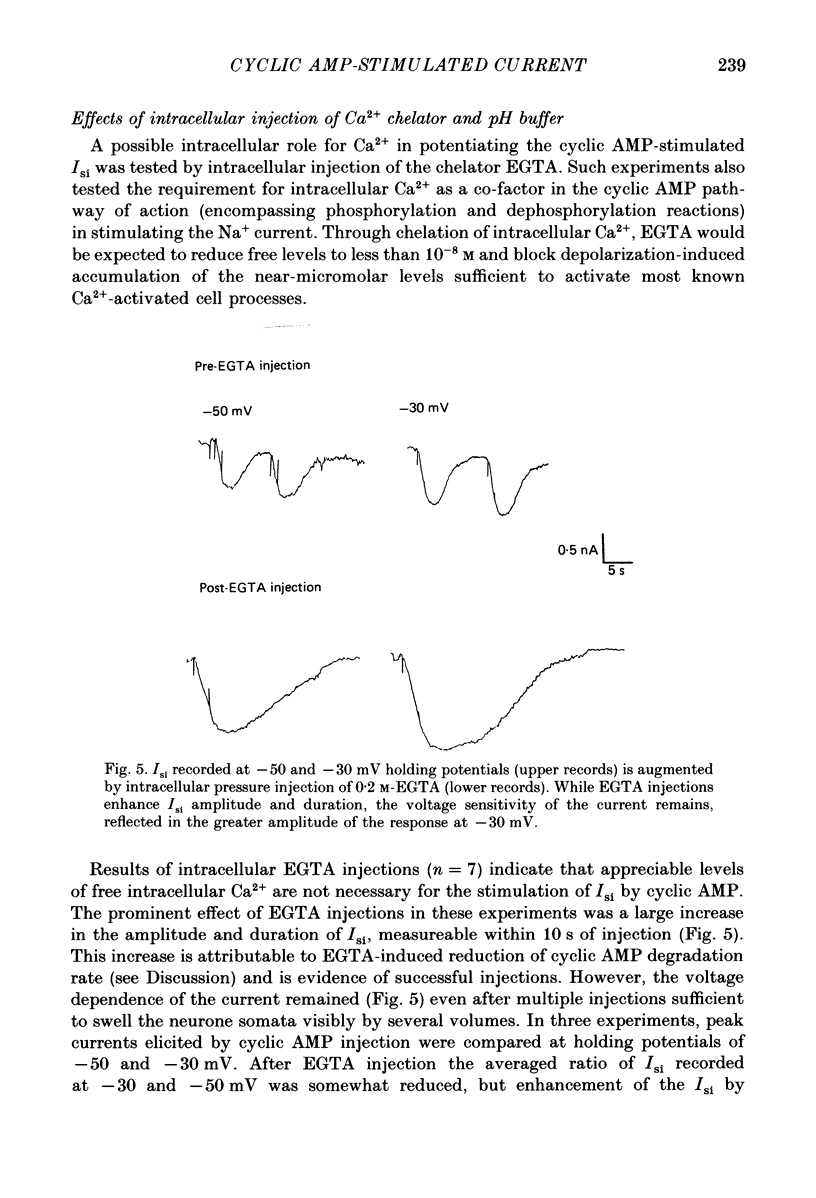
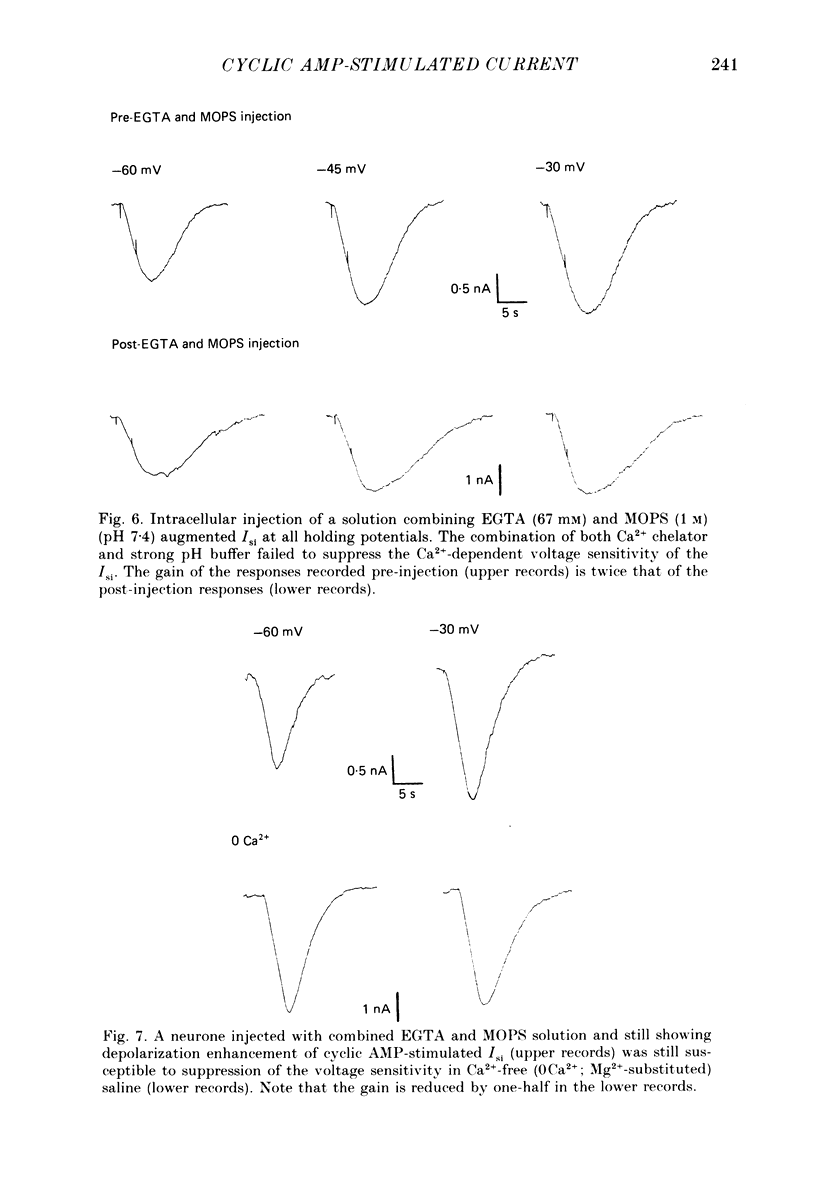
1. Ionophoretic injection of cyclic AMP into a voltage-clamped molluscan neurone caused a transient slow inward current (Isi) whose amplitude was enhanced by depolarization. Na+-replaced salines abolished the current, placing it with cyclic AMP-stimulated Na+ currents of other gastropod species. 2. Isi amplitude was suppressed by extracellular Ca2+. The amplitude increased up to 4-fold at holding potentials of -50 mV in nominally Ca2+-free saline. Ion substitutions showed that Ca2+ suppressed Isi more effectively than Mg2+, Co2+, Cd2+, Mn2+, Ba2+ or Sr2+. 3. Voltage sensitivity of Isi was abolished by low-Ca2+ salines, by the Ca2+ current blocker Co2+ and by substitution of Ba2+ or Sr2+ as Ca2+ channel current carriers. In such salines Isi showed no appreciable change in amplitude at holding potentials between -70 and -25 mV. 4. Intracellular injection of the Ca2+ chelator EGTA both augmented the amplitude of the current and its duration. EGTA injection failed to suppress the Ca2+-dependent voltage sensitivity of Isi. Intracellular injection of concentrated 3-N-(morpholino) propanesulphonic acid (MOPS) pH buffer to inhibit secondary, Ca2+-dependent intracellular acidification also failed to suppress the voltage sensitivity, as did injections of a mixed EGTA and MOPS solution. 5. While the data indicate a requirement for extracellular Ca2+ in conferring voltage sensitivity, they do not support a role for an intracellular action. An extracellular binding site for Ca2+ could mediate the voltage sensitivity, either by local depolarization-dependent changes in extracellular Ca2+ concentration or through direct voltage-sensitive block of the Isi channel.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. J Gen Physiol. 1980 Apr;75(4):403–426. doi: 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenhoff J. B., Hofmeier G., Lux H. D., Swandulla D. Stimulation of a sodium influx by cAMP in Helix neurons. Brain Res. 1983 Oct 16;276(2):289–296. doi: 10.1016/0006-8993(83)90736-9. [DOI] [PubMed] [Google Scholar]
- Calhoon R. D., Gillette R. Ca2+ activated and pH sensitive cyclic AMP phosphodiesterase in the nervous system of the mollusc Pleurobranchaea. Brain Res. 1983 Jul 25;271(2):371–374. doi: 10.1016/0006-8993(83)90304-9. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Hockberger P. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones. J Physiol. 1984 Sep;354:139–162. doi: 10.1113/jphysiol.1984.sp015368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald D. A., Williams A., Levitan I. B. Modulation of single Ca2+-dependent K+-channel activity by protein phosphorylation. Nature. 1985 Jun 6;315(6019):503–506. doi: 10.1038/315503a0. [DOI] [PubMed] [Google Scholar]
- Gillette M. U., Gillette R. Bursting neurons command consummatory feeding behavior and coordinated visceral receptivity in the predatory mollusk Pleurobranchaea. J Neurosci. 1983 Sep;3(9):1791–1806. doi: 10.1523/JNEUROSCI.03-09-01791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R., Gillette M. U., Davis W. J. Action-potential broadening and endogenously sustained bursting are substrates of command ability in a feeding neuron of Pleurobranchaea. J Neurophysiol. 1980 Mar;43(3):669–685. doi: 10.1152/jn.1980.43.3.669. [DOI] [PubMed] [Google Scholar]
- Gillette R., Green D. J. Phenothiazines mimic the action of cAMP in potentiating slow inward current in a bursting molluscan neuron. Brain Res. 1983 Aug 29;273(2):384–386. doi: 10.1016/0006-8993(83)90868-5. [DOI] [PubMed] [Google Scholar]
- Gillette R. Intracellular alkalinization potentiates slow inward current and prolonged bursting in a molluscan neuron. J Neurophysiol. 1983 Feb;49(2):509–515. doi: 10.1152/jn.1983.49.2.509. [DOI] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Patch- and voltage-clamp analysis of cyclic AMP-stimulated inward current underlying neurone bursting. Nature. 1983 Dec 22;306(5945):784–785. doi: 10.1038/306784a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. D., Scheller R. H. Egg-laying hormone of Aplysia induces a voltage-dependent slow inward current carried by Na+ in an identified motoneuron. Proc Natl Acad Sci U S A. 1986 May;83(9):3017–3021. doi: 10.1073/pnas.83.9.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko N. I., Kostyuk P. G., Shcherbatko A. D. The effect of intracellular cAMP injections on stationary membrane conductance and voltage- and time-dependent ionic currents in identified snail neurons. Brain Res. 1983 Jun 6;268(2):321–338. doi: 10.1016/0006-8993(83)90499-7. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-dependent inward current in Aplysia bursting pace-maker neurones. J Physiol. 1985 May;362:107–130. doi: 10.1113/jphysiol.1985.sp015666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-induced inactivation of calcium current causes the inter-burst hyperpolarization of Aplysia bursting neurones. J Physiol. 1985 May;362:131–160. doi: 10.1113/jphysiol.1985.sp015667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Lux H. D. Changes in ionic conductances induced by cAMP in Helix neurons. Brain Res. 1984 Jul 2;305(1):115–122. doi: 10.1016/0006-8993(84)91126-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


