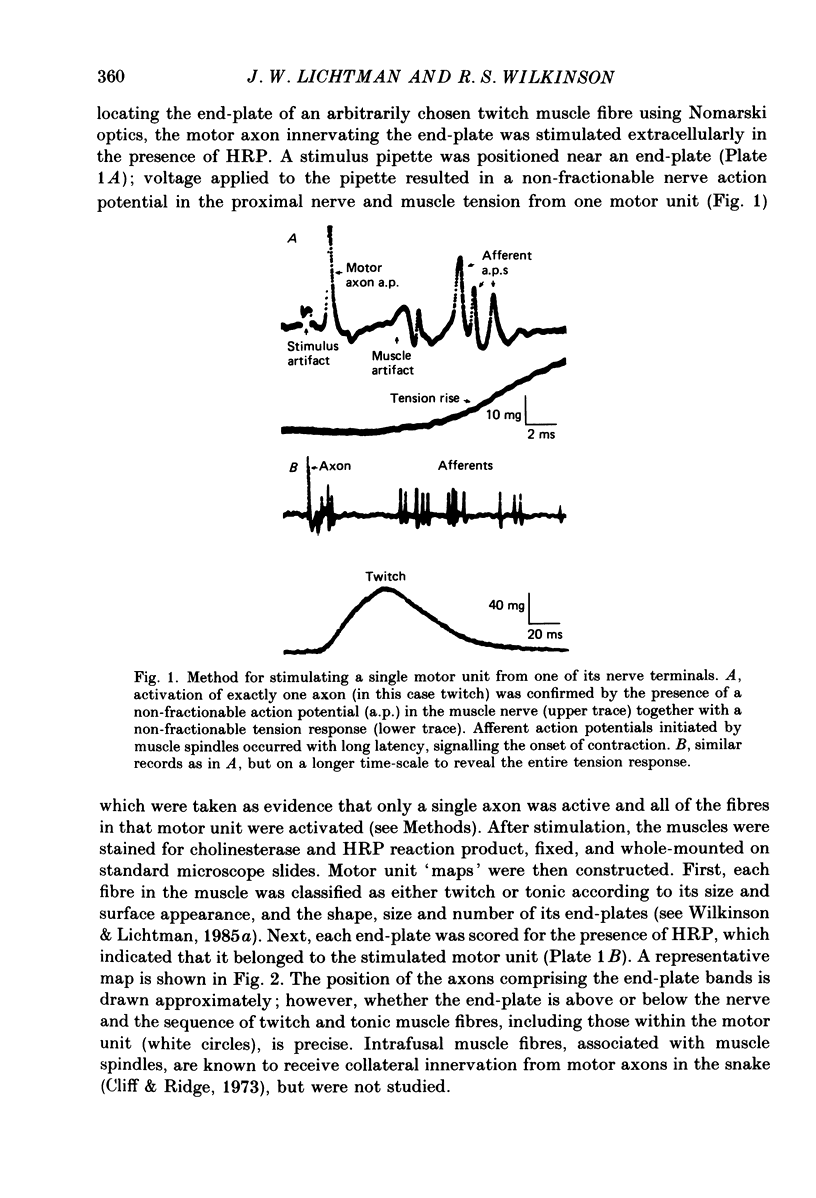
Abstract
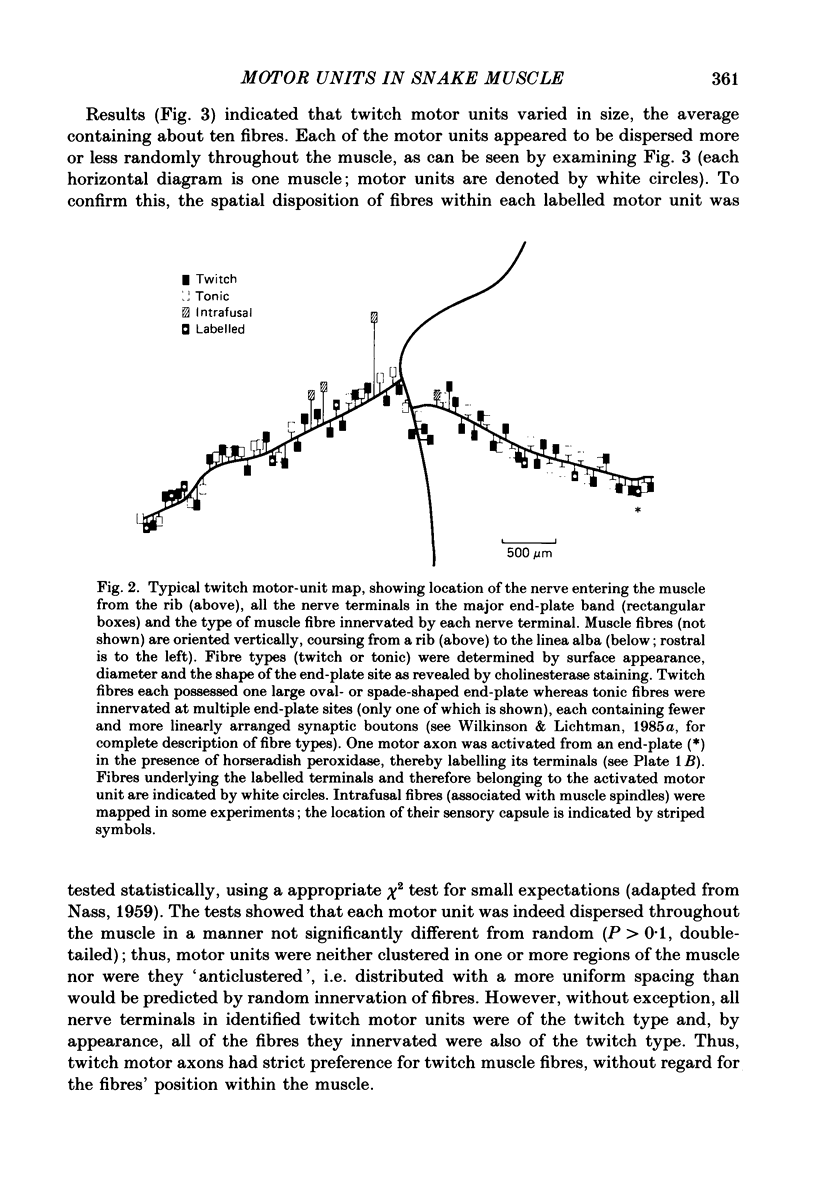
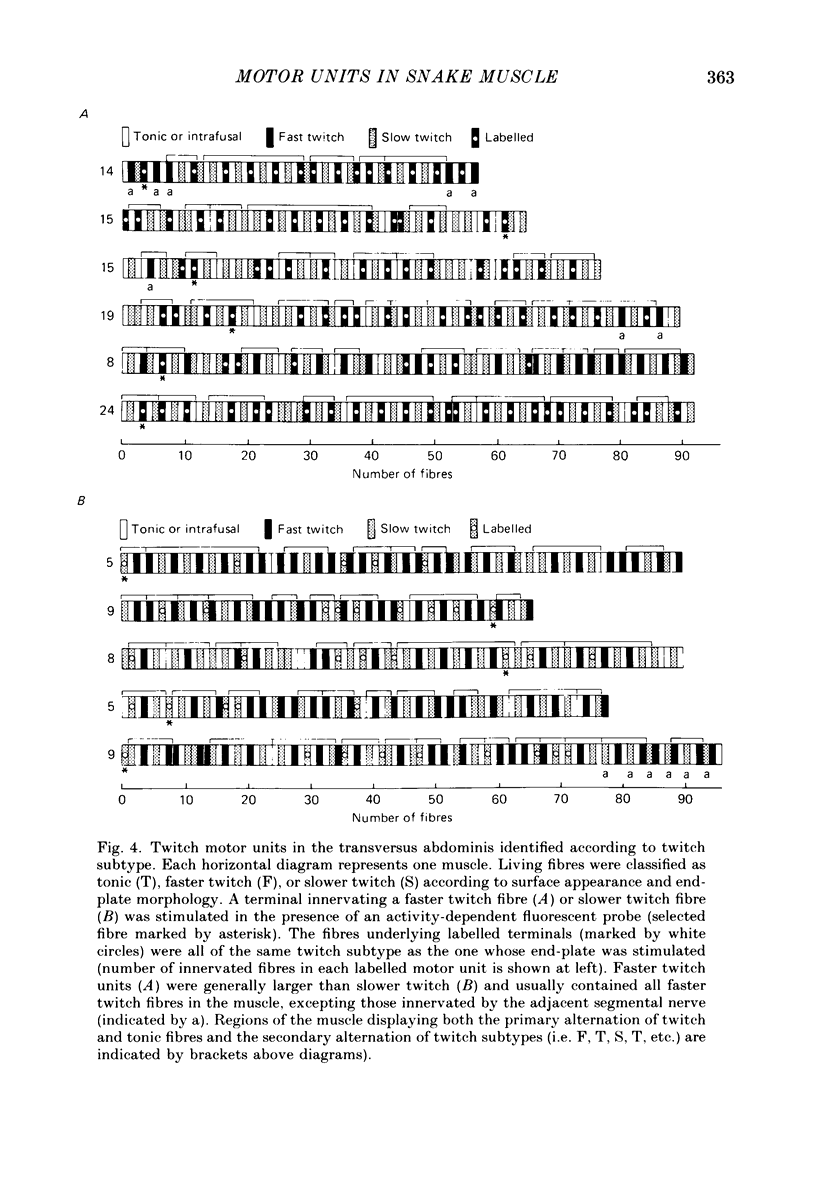
1. The organization of motor units in the single-fibre-thick transversus abdominis muscle of the garter snake has been studied. This small segmental muscle (60-100 fibres) contains three distinct fibre types (faster twitch, F; slower twitch, S; and tonic, T) which are predominantly arranged in the repeating pattern F, T, S, T, F, T, etc. 2. Motor-unit maps were obtained by activating an individual motor axon and identifying all of the muscle fibres innervated by that axon, using either the activity-induced uptake of extracellular marker molecules to label presynaptic terminals of the stimulated axon, or systematic intracellular recording to identify muscle fibres activated by the axon. 3. Each muscle contained three types of motor units (F, T and S) that corresponded to the three types of fibres. All of the muscle fibres in a motor unit were of the same type. Each segmental muscle contained approximately the same number of motor units: one to two faster twitch, three to four slower twitch, and three tonic. 4. Each motor unit was dispersed widely throughout the muscle. Fibres within a motor unit were neither clustered nor anticlustered. This suggests that despite the fact that axons are constrained to innervate fibres of the appropriate type, the distribution of each motor unit does not generate the alternating pattern of fibre types. 5. In several experiments, all of the twitch motor units in one segmental component of the muscle were mapped. The projection of any one axon appeared random not only with respect to the muscle's fibre type pattern, but also with respect to the innervation supplied by other axons. 6. Twitch motor units were arranged according to a hierarchy of sizes. In each muscle examined, the largest motor unit was faster twitch; a single faster twitch motor axon usually innervated all of the faster twitch fibres in the muscle (fourteen to twenty-four fibres). This was followed by three to four slower twitch motor units which varied in size from eight to ten fibres to very small motor units containing only four to five fibres. 7. Each tonic motor axon innervated an average of ninety-three end-plates per segmental muscle. The relatively large size of tonic motor units compared to twitch motor units is related to the ability of tonic muscle fibres to retain polyneuronal innervation into adulthood, both by providing five to seven end-plate sites per fibre, and by allowing terminal boutons from different tonic motor axons to co-innervate the same end-plate.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






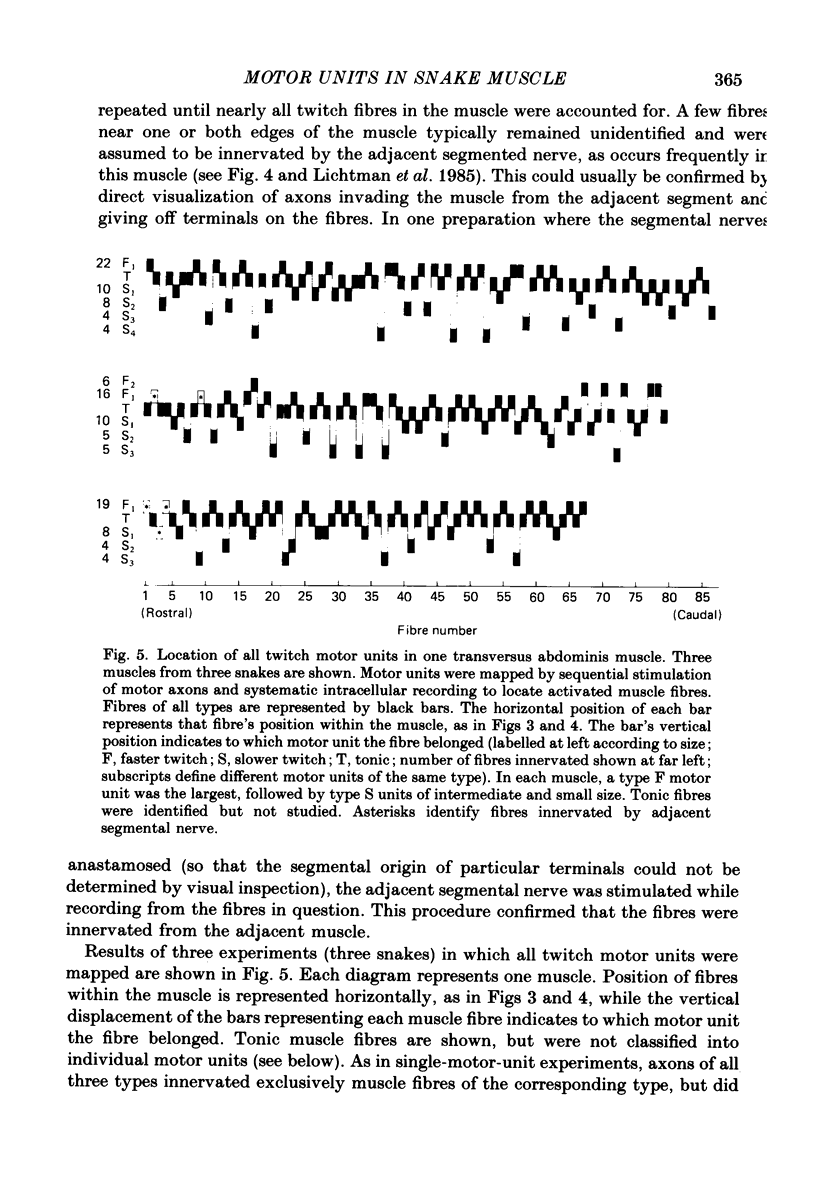










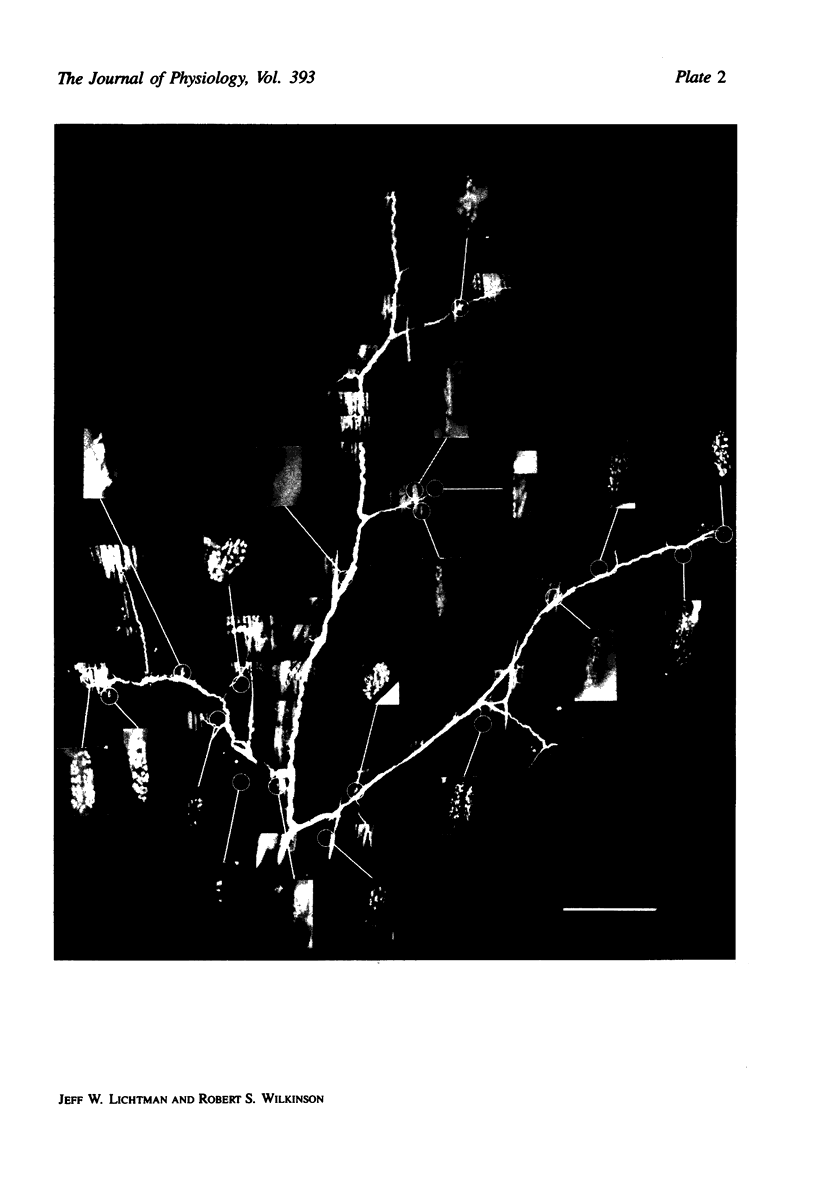
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., ECCLES J. C., ECCLES R. M. Differentiation of fast and slow muscles in the cat hind limb. J Physiol. 1960 Feb;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi A. Y., Chiarandini D. J. Intracellular marking of skeletal muscle cells with horseradish peroxidase in combination with a stain for cholinesterase. Stain Technol. 1980 Mar;55(2):105–109. [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Butler J., Cosmos E., Brierley J. Differentiation of muscle fiber types in aneurogenic brachial muscles of the chick embryo. J Exp Zool. 1982 Nov 20;224(1):65–80. doi: 10.1002/jez.1402240108. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff G. S., Ridge R. M. Innervation of extrafusal and intrafusal fibres in snake muscle. J Physiol. 1973 Aug;233(1):1–18. doi: 10.1113/jphysiol.1973.sp010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E. Relation between size of neurons and their susceptibility to discharge. Science. 1957 Dec 27;126(3287):1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hammond G. R., Ridge R. M. Properties of twitch motor units in snake costocutaneous muscle. J Physiol. 1978 Mar;276:525–533. doi: 10.1113/jphysiol.1978.sp012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. F. Neural regulation of mammalian fast and slow muscle myosins: an electrophoretic analysis. Biochemistry. 1975 Feb 25;14(4):742–747. doi: 10.1021/bi00675a015. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. The non-selective innervation of muscle fibres and mixed composition of motor units in a muscle of neonatal rat. J Physiol. 1987 May;386:377–394. doi: 10.1113/jphysiol.1987.sp016539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. "Type grouping" in skeletal muscles after experimental reinnervation. Neurology. 1968 May;18(5):447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Rubinstein N. A. Development of neuromuscular specialization. Med Sci Sports Exerc. 1986 Jun;18(3):292–298. doi: 10.1249/00005768-198606000-00007. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L., Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing N. G., Lamb A. H. The distribution of muscle fibre types in chick embryo wings transplanted to the pelvic region is normal. J Embryol Exp Morphol. 1983 Dec;78:67–82. [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S., Rich M. M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. 1985 Mar 28-Apr 3Nature. 314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- Maddox J. Natural history of yellow rain. Nature. 1984 May 17;309(5965):207–207. doi: 10.1038/309207a0. [DOI] [PubMed] [Google Scholar]
- Michaels R. L. Immunocytochemical identification of dye-injected cells. J Histochem Cytochem. 1982 Feb;30(2):189–191. doi: 10.1177/30.2.7037940. [DOI] [PubMed] [Google Scholar]
- Morgan D. L., Proske U. Non-linear summation of tension in motor units of toad slow muscle. J Physiol. 1984 Apr;349:95–105. doi: 10.1113/jphysiol.1984.sp015145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L., Proske U. Vertebrate slow muscle: its structure, pattern of innervation, and mechanical properties. Physiol Rev. 1984 Jan;64(1):103–169. doi: 10.1152/physrev.1984.64.1.103. [DOI] [PubMed] [Google Scholar]
- Nemeth P. M., Pette D., Vrbová G. Comparison of enzyme activities among single muscle fibres within defined motor units. J Physiol. 1981 Feb;311:489–495. doi: 10.1113/jphysiol.1981.sp013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Regulation of synaptic position, size, and strength in anuran skeletal muscle. J Neurosci. 1983 Jan;3(1):161–176. doi: 10.1523/JNEUROSCI.03-01-00161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Phillips W. D., Bennett M. R. Differentiation of fiber types in wing muscles during embryonic development: effect of neural tube removal. Dev Biol. 1984 Dec;106(2):457–468. doi: 10.1016/0012-1606(84)90245-8. [DOI] [PubMed] [Google Scholar]
- Ridge R. M. Different types of extrafusal muscle fibres in snake costocutaneous muscles. J Physiol. 1971 Sep;217(2):393–418. doi: 10.1113/jphysiol.1971.sp009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Development of muscle fiber specialization in the rat hindlimb. J Cell Biol. 1981 Jul;90(1):128–144. doi: 10.1083/jcb.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. S., Lichtman J. W. Regular alternation of fiber types in the transversus abdominis muscle of the garter snake. J Neurosci. 1985 Nov;5(11):2979–2988. doi: 10.1523/JNEUROSCI.05-11-02979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]