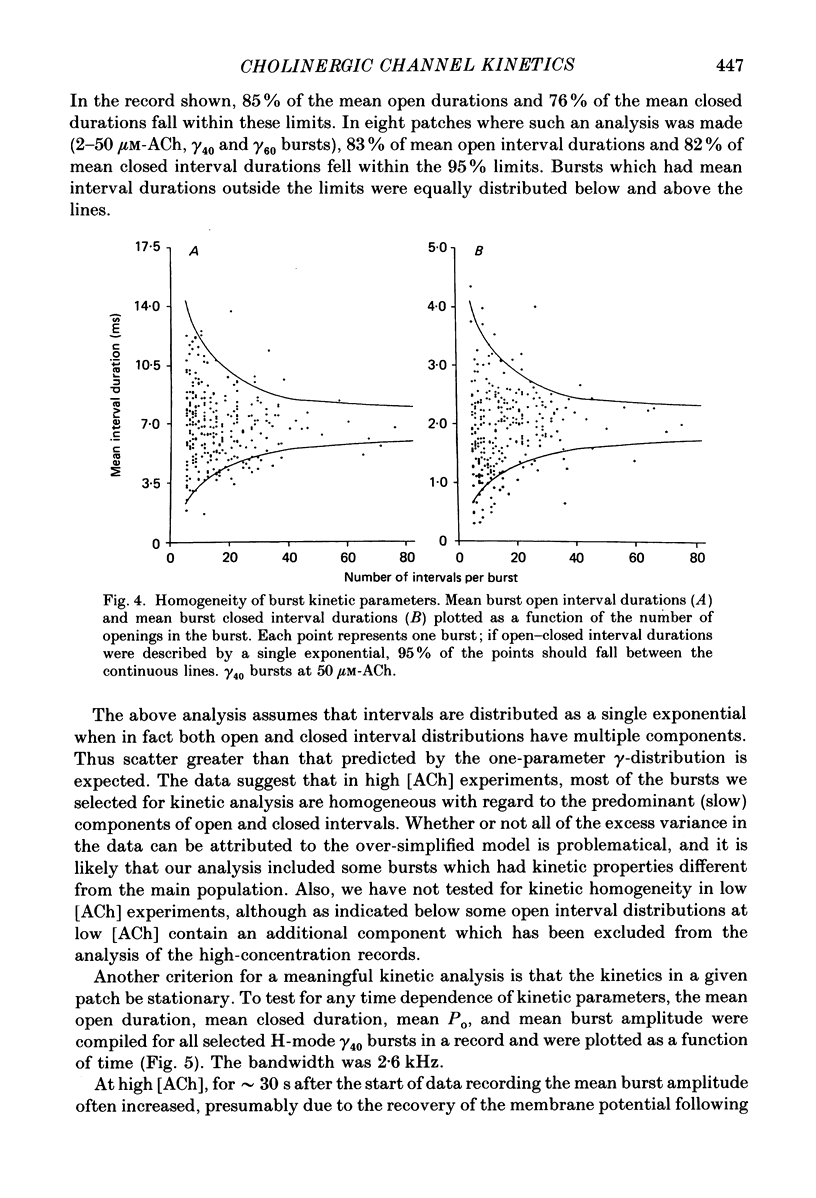
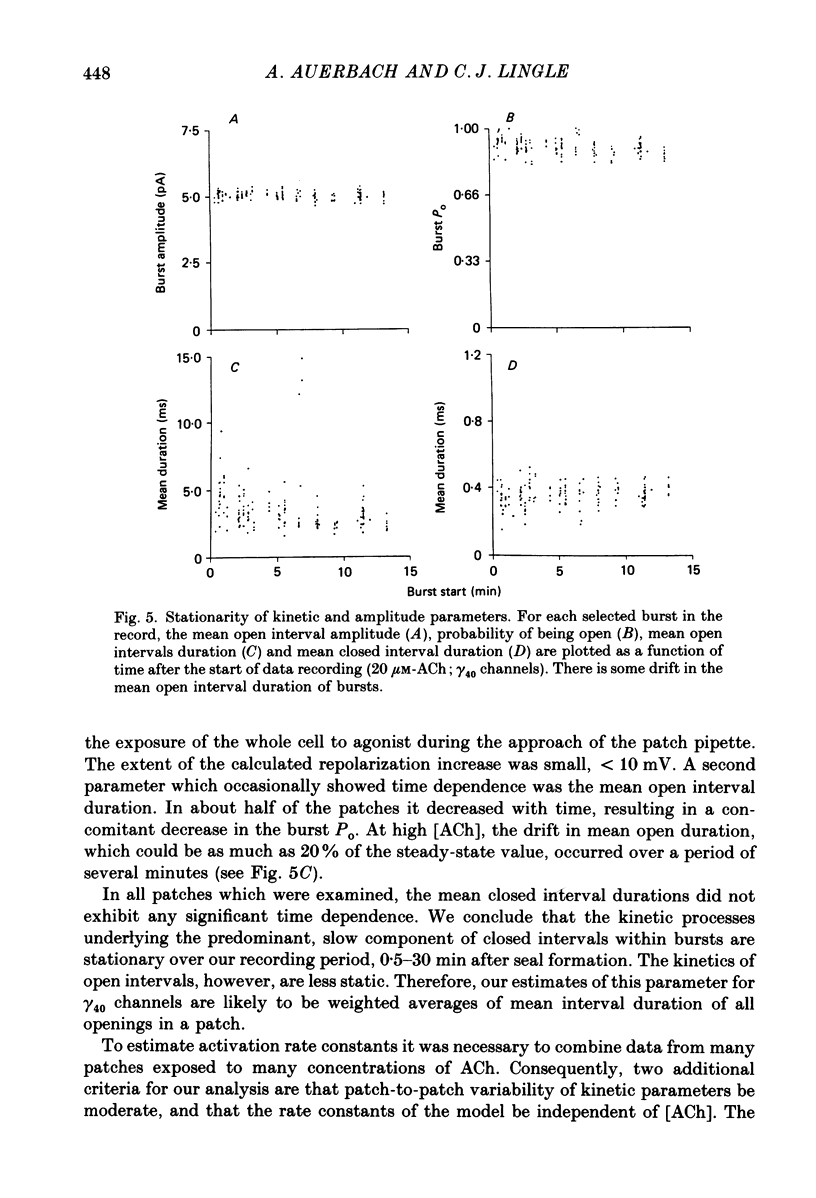
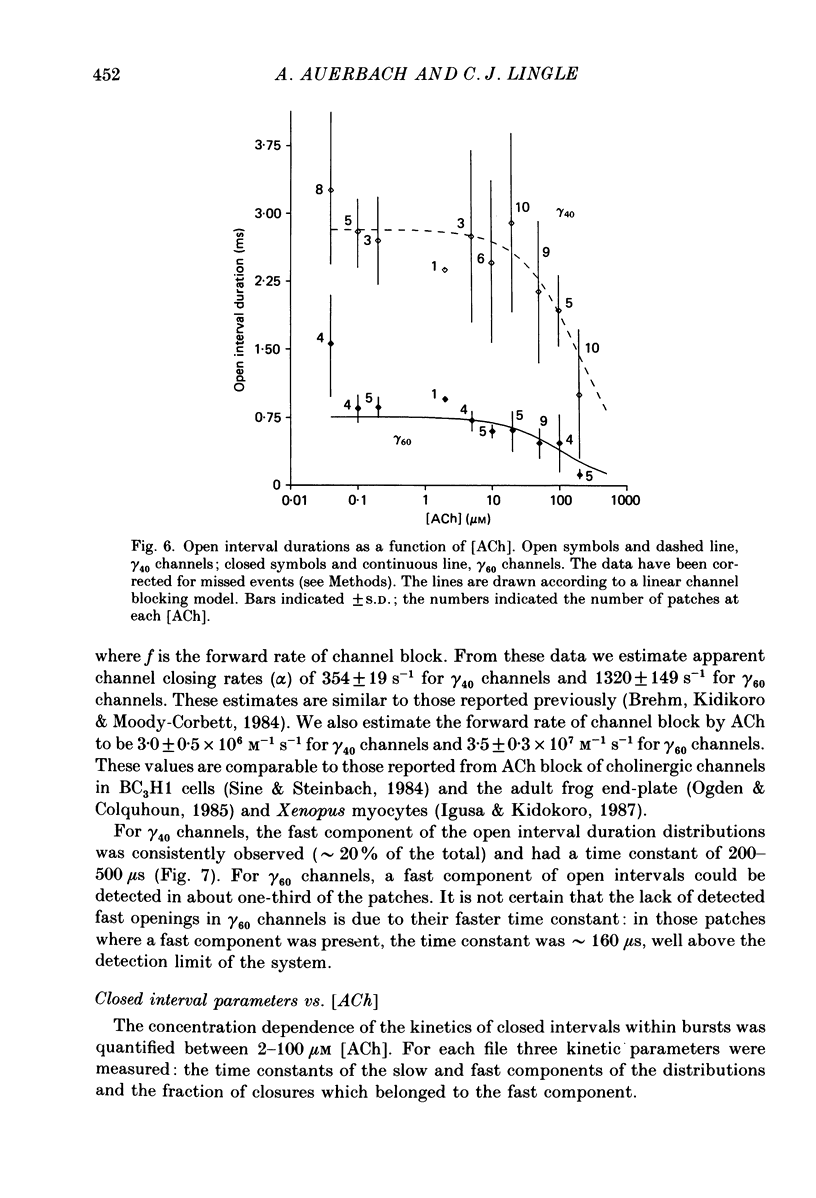
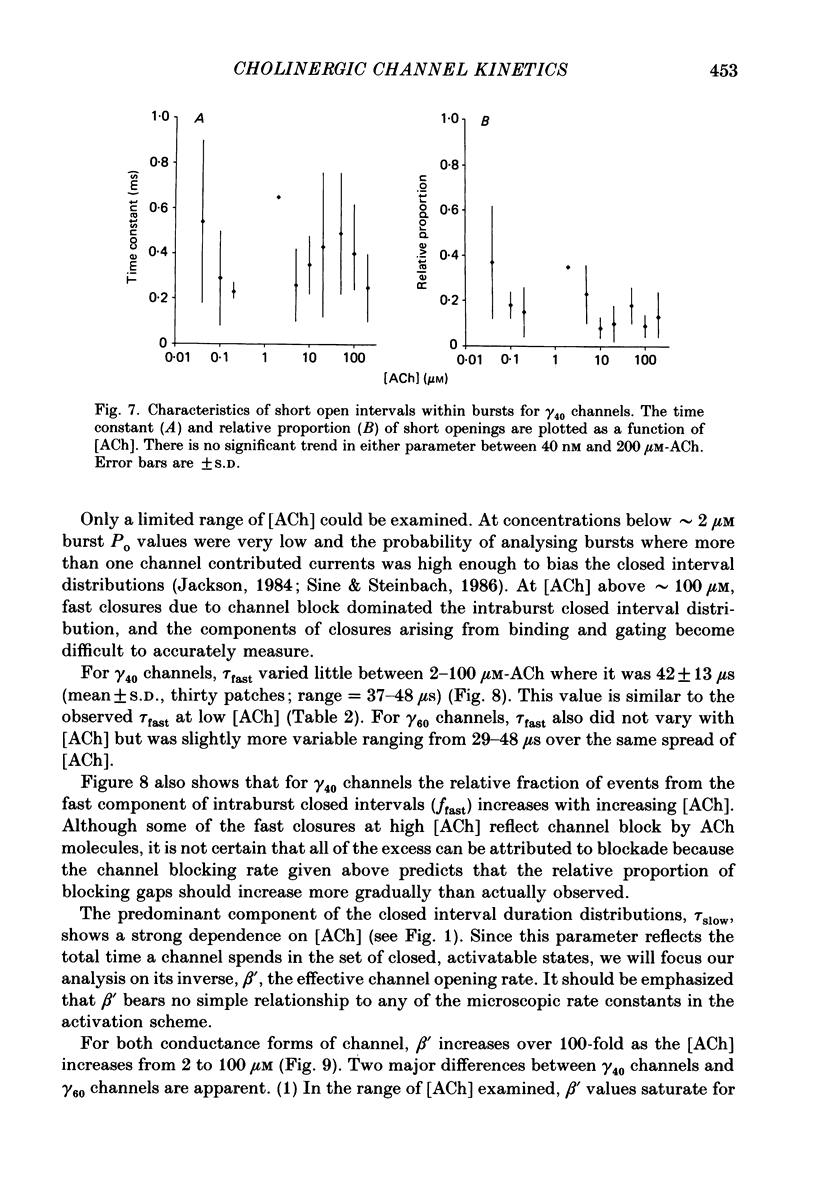
Abstract
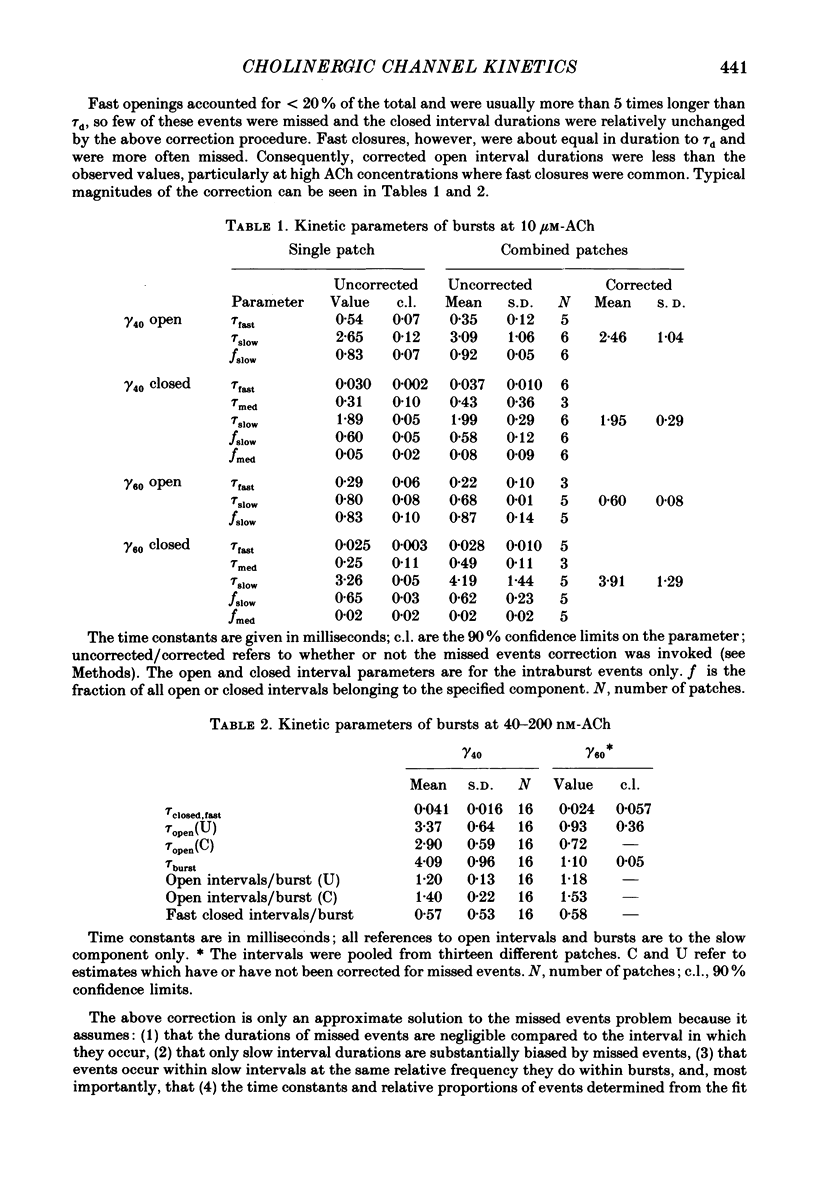
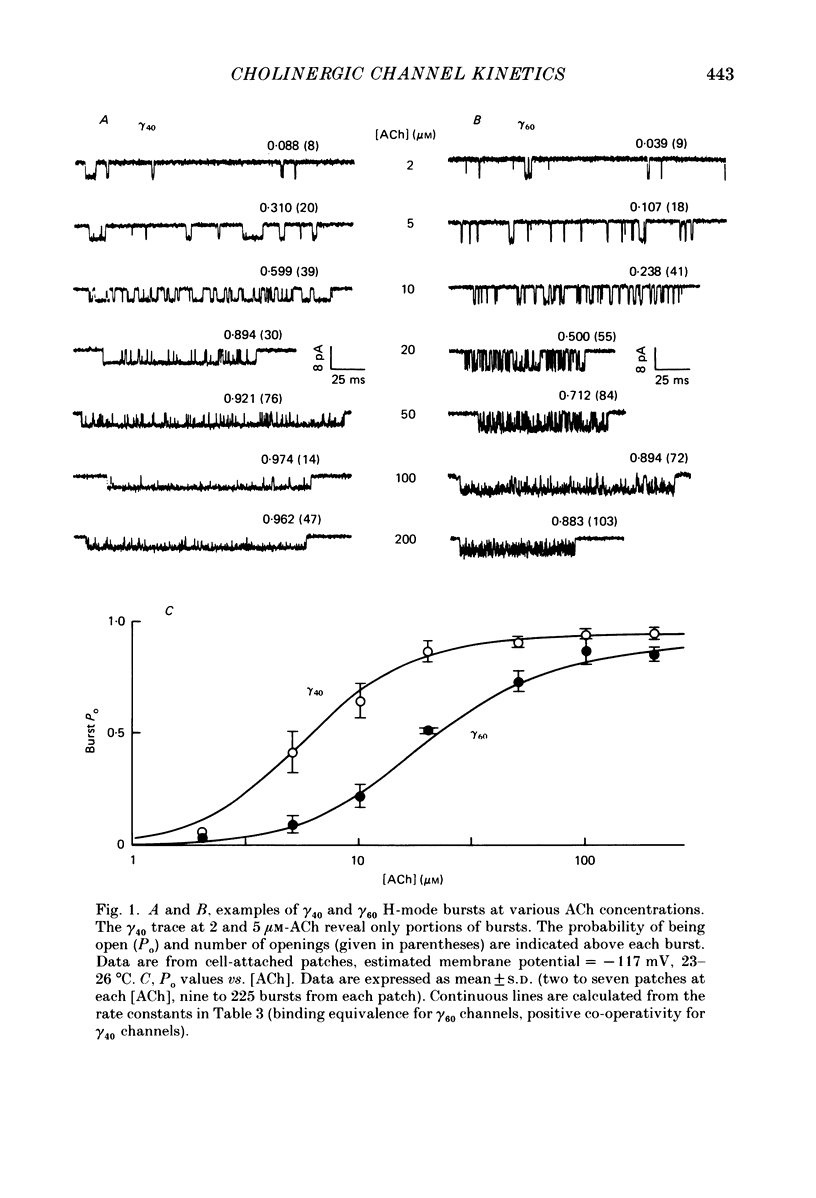
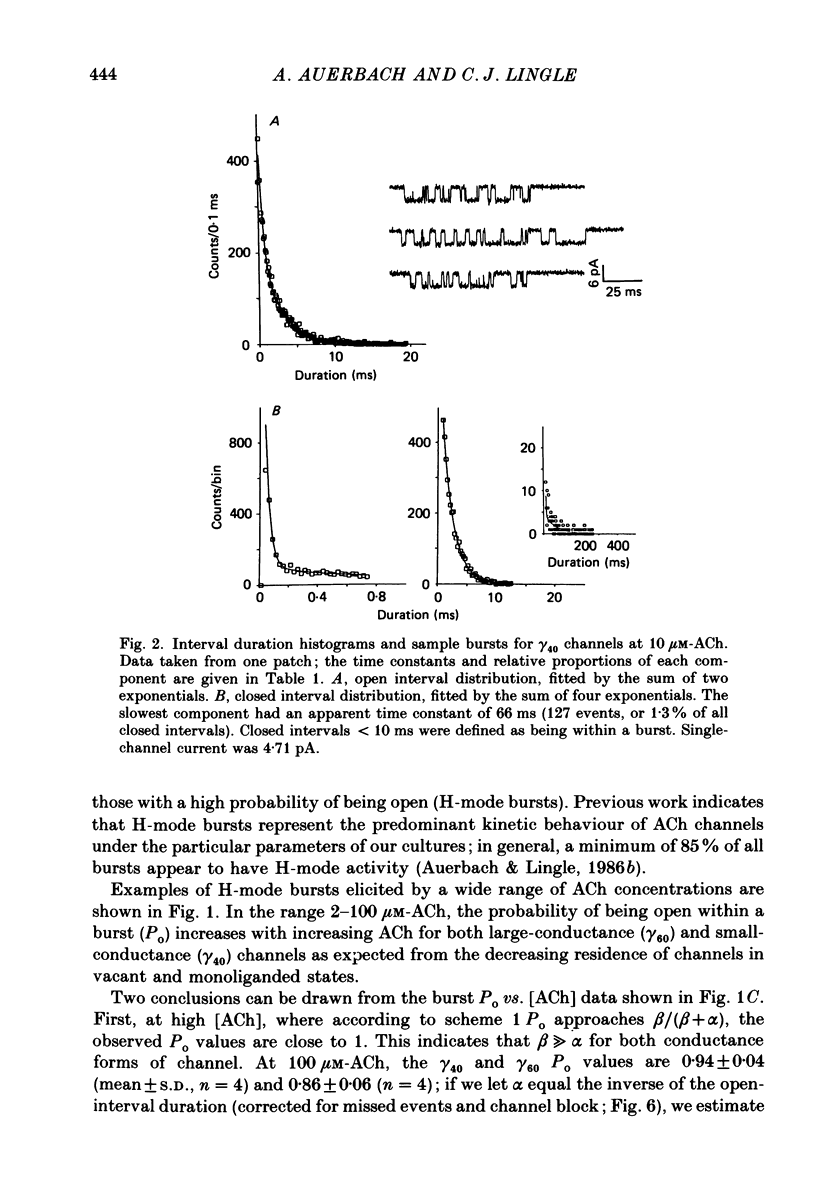
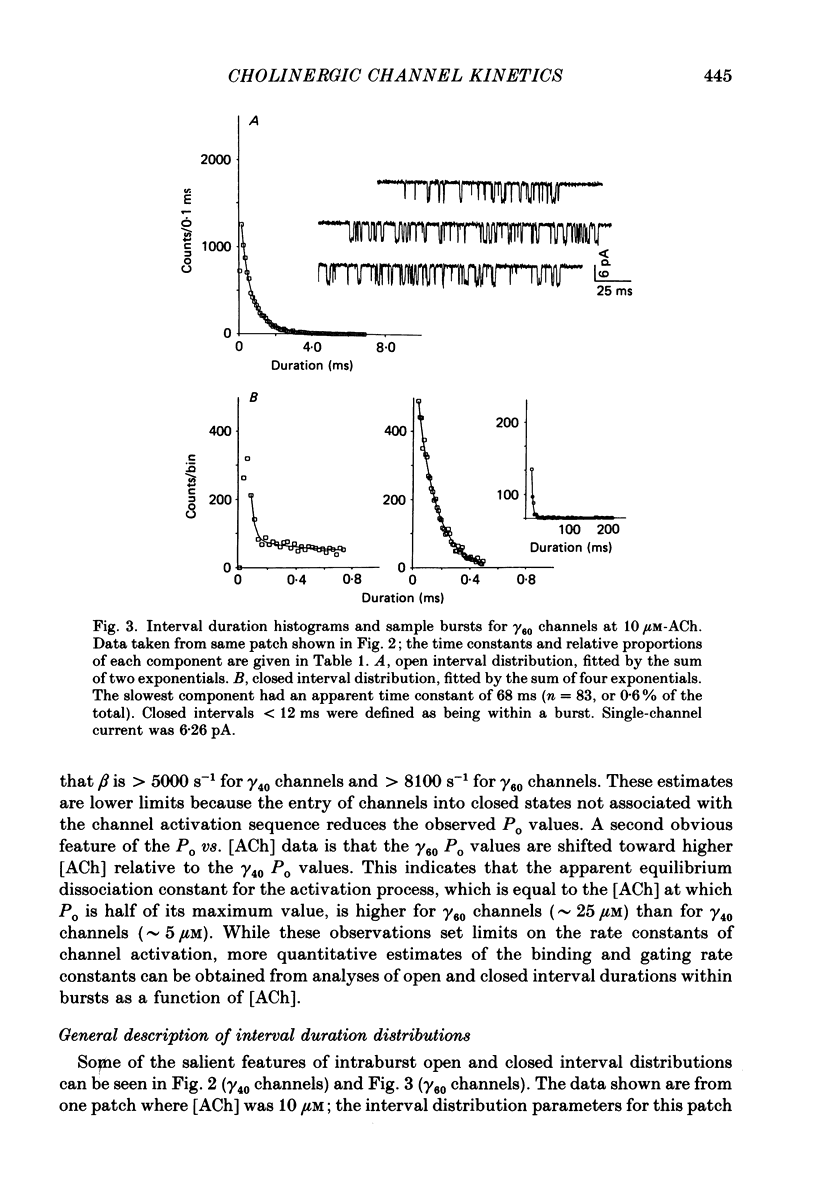
1. The kinetic properties of single acetylcholine (ACh)-activated ion channels in tissue-cultured Xenopus myocytes have been examined in cell-attached patches. The rates of agonist binding and channel gating were inferred from the durations of open and closed intervals from channels exposed to 40 nM-200 microM-ACh. The predominant kinetic forms of large- (gamma 60) and small-conductance (gamma 40) cholinergic channels were compared. 2. At high [ACh], bursts were defined so that they primarily reflect sojourns in activatable states. The probability that a channel is open within a burst (Po) increases between 2 and 200 microM-ACh. Po is half-maximal at approximately 5 microM for gamma 40 channels and at approximately 25 microM for gamma 60 channels. 3. Open interval durations for gamma 40 channels are distributed as the sum of two exponentials, with the slow component (tau approximately 2.8 ms) accounting for greater than 80% of the total. Open interval durations for gamma 60 channels are often distributed as a single exponential with an apparent time constant of approximately 0.8 ms. For both conductance forms of channel, open interval durations show no significant dependence on [ACh] in the range 0.04-10 microM, but decrease at higher [ACh] in a manner consistent with channel block by agonist molecules. 4. Closed interval durations within bursts (2-100 microM-ACh) for gamma 60 or gamma 40 channels are described by the sum of two or three exponentials. For both conductance forms of channel the apparent time constant of the fastest component is approximately 40 microseconds and does not change significantly with [ACh], and the time constant of the predominant, slowest component (tau slow) decreases with increasing [ACh]. 5. For gamma 40 channels, at high [ACh] tau slow saturates at approximately 0.17 ms, while no saturation is apparent for gamma 60 channel tau slow values up to 200 microM-ACh. Below 50 microM-ACh, gamma 40 tau slow values are approximately 1.5 times shorter than gamma 60 values. 6. Estimates of rate constants for agonist binding and channel gating were obtained by fitting closed interval durations in the range 2-100 microM-ACh. Gamma 60 channels have a greater than 5-fold faster opening rate, approximately 10-fold faster closing rate, and approximately 3-fold lower affinity than do gamma 40 channels. There is some indication of positive co-operativity of ACh binding to gamma 40 channels.
Full text
PDF









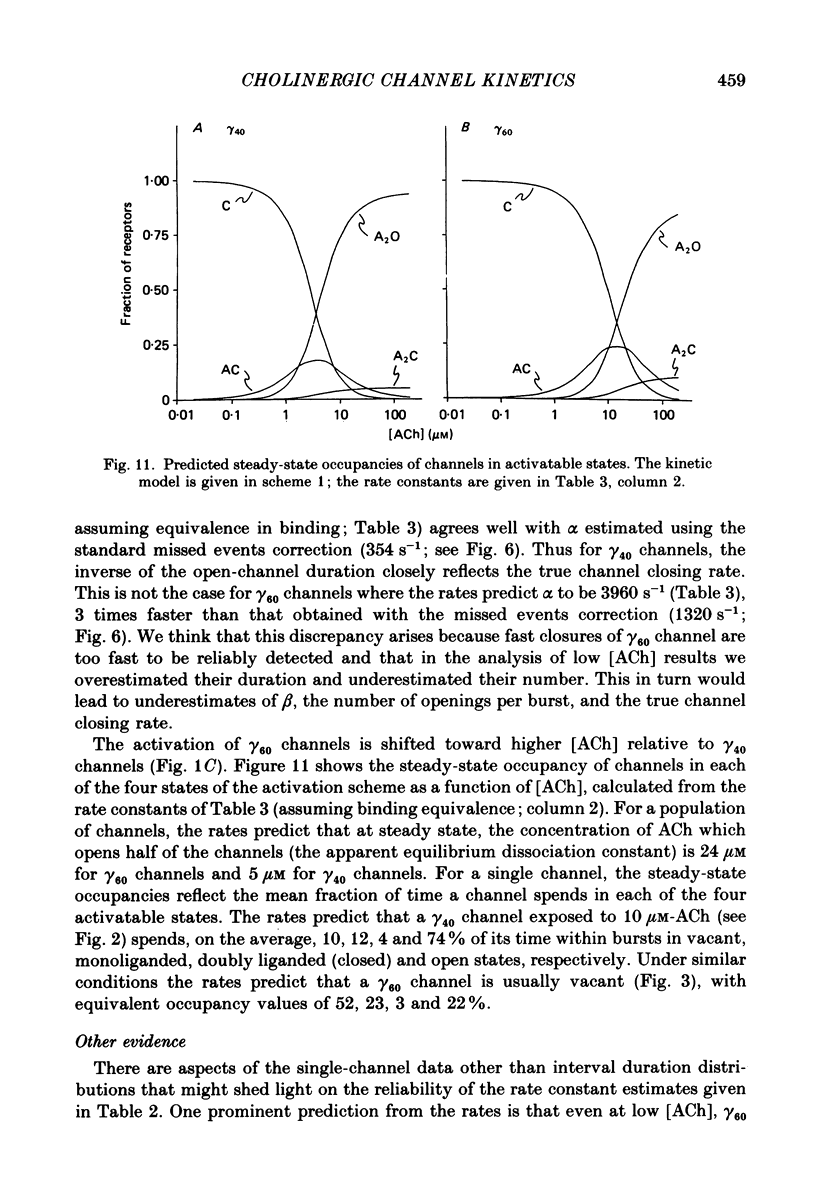
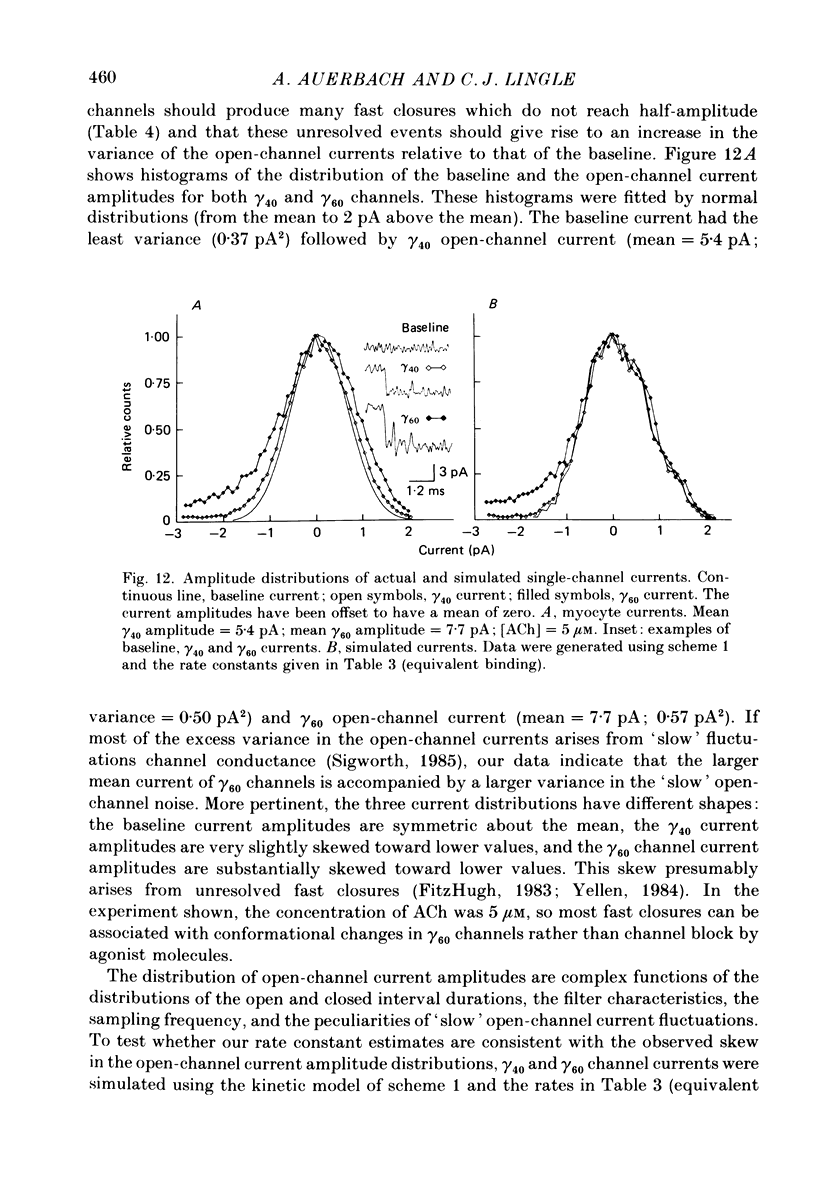






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Single-channel currents from acetylcholine receptors in embryonic chick muscle. Kinetic and conductance properties of gaps within bursts. Biophys J. 1984 Jan;45(1):187–198. doi: 10.1016/S0006-3495(84)84147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Kidokoro Y., Moody-Corbett F. Acetylcholine receptor channel properties during development of Xenopus muscle cells in culture. J Physiol. 1984 Dec;357:203–217. doi: 10.1113/jphysiol.1984.sp015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Steinbach J. H., Kidokoro Y. Channel open time of acetylcholine receptors on Xenopus muscle cells in dissociated cell culture. Dev Biol. 1982 May;91(1):93–102. doi: 10.1016/0012-1606(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Brett R. S., Dilger J. P., Adams P. R., Lancaster B. A method for the rapid exchange of solutions bathing excised membrane patches. Biophys J. 1986 Nov;50(5):987–992. doi: 10.1016/S0006-3495(86)83539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Dwyer T. M. The rising phase of the miniature endplate current at the frog neuromuscular junction. Biochim Biophys Acta. 1981 Aug 6;646(1):51–60. doi: 10.1016/0005-2736(81)90271-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Igusa Y., Kidokoro Y. Two types of acetylcholine receptor channels in developing Xenopus muscle cells in culture: further kinetic analyses. J Physiol. 1987 Aug;389:271–300. doi: 10.1113/jphysiol.1987.sp016657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Spontaneous openings of the acetylcholine receptor channel. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3901–3904. doi: 10.1073/pnas.81.12.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R., Owens J. L., Vickers J. Development of synaptic currents in immobilized muscle of Xenopus laevis. J Physiol. 1985 Jul;364:57–68. doi: 10.1113/jphysiol.1985.sp015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. D., Dionne V. E. Single-channel acetylcholine receptor kinetics. Biophys J. 1984 Jan;45(1):153–163. doi: 10.1016/S0006-3495(84)84144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Nakajima S., Nakajima Y., Takahashi T. Differential development of two classes of acetylcholine receptors in Xenopus muscle in culture. Science. 1984 Oct 5;226(4670):55–57. doi: 10.1126/science.6474189. [DOI] [PubMed] [Google Scholar]
- Marshall L. M. Presynaptic control of synaptic channel kinetics in sympathetic neurones. Nature. 1985 Oct 17;317(6038):621–623. doi: 10.1038/317621a0. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Sachs F. Single ionic channels observed in tissue-cultured muscle. Nature. 1979 Dec 20;282(5741):861–863. doi: 10.1038/282861a0. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Auerbach A. Single-channel electrophysiology: use of the patch clamp. Methods Enzymol. 1983;103:147–176. doi: 10.1016/s0076-6879(83)03011-6. [DOI] [PubMed] [Google Scholar]
- Sachs F., Lecar H. Acetylcholine-induced current fluctuations in tissue-cultured muscle cells under voltage clamp. Biophys J. 1977 Feb;17(2):129–143. doi: 10.1016/S0006-3495(77)85631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Open channel noise. I. Noise in acetylcholine receptor currents suggests conformational fluctuations. Biophys J. 1985 May;47(5):709–720. doi: 10.1016/S0006-3495(85)83968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


