Abstract
1. The whole-cell variation of the patch clamp was used to study ion channel properties in the unfertilized oocyte, and in surgically isolated blastomeres from 2-, 4-, and 8-cell embryos of the ascidian, Boltenia villosa. 2. The unfertilized oocyte has three major voltage-dependent currents: a transient, inward Na+ current; a transient, inward Ca2+ current; and an inwardly rectifying K+ current. 3. The total surface area of the embryo, either measured by capacitance or calculated from cell diameters, increased about 2.5-fold between fertilization and the 8-cell stage. 4. The Na+ current almost completely disappeared from the embryo by the time of first cleavage and was undetectable in any of the blastomeres at the 8-cell stage. This loss was too large to be explained by the dilution of channels in the oocyte due to newly added membrane. 5. Both the Ca2+ current and the inwardly rectifying K+ current were maintained at constant or slightly increased density through the first three cleavage cycles. This suggests that these channels are added along with new membrane during these stages. 6. No differences in mean current densities of blastomeres of different developmental fates were detected through the 8-cell stage. 7. Continuous recordings in single egg cells between fertilization and first cleavage, using two-microelectrode voltage clamp, revealed the increase in capacitance, Ca2+ current amplitude, and K+ current amplitude, and the loss of Na+ current predicted from the blastomere studies.
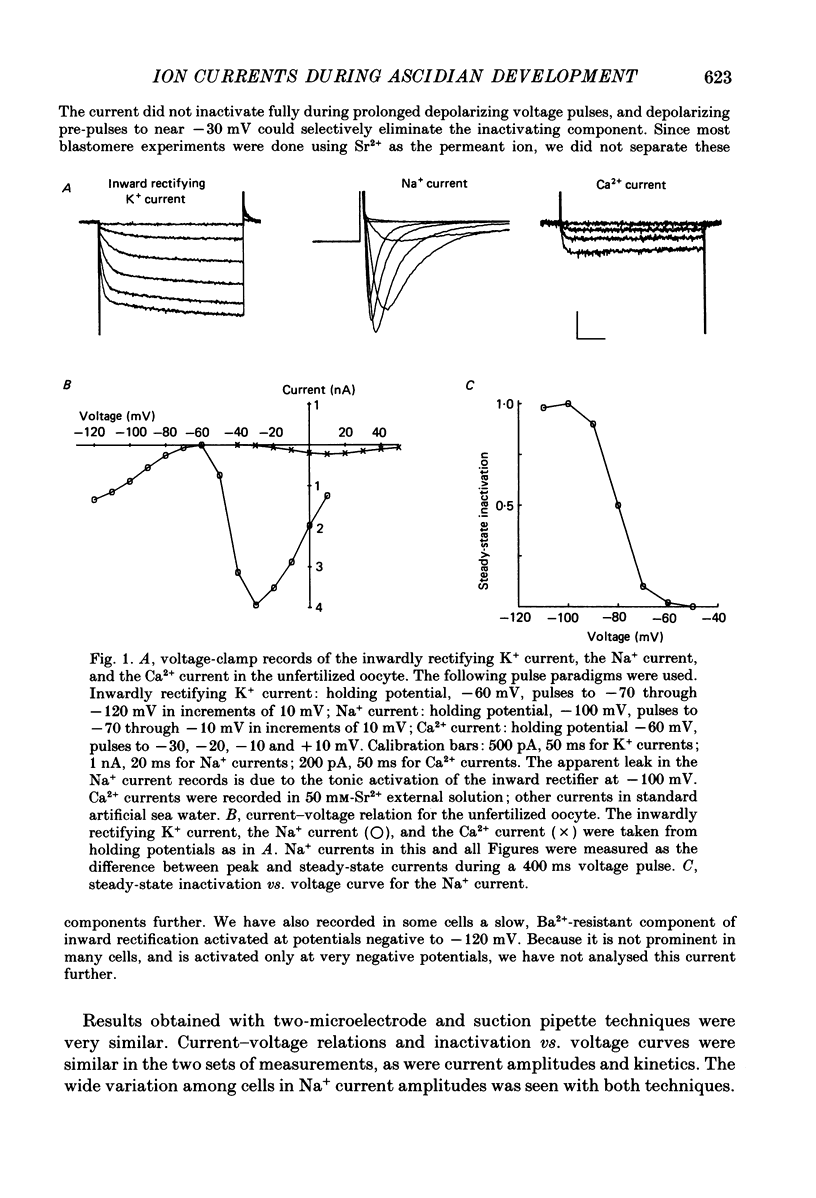
Full text
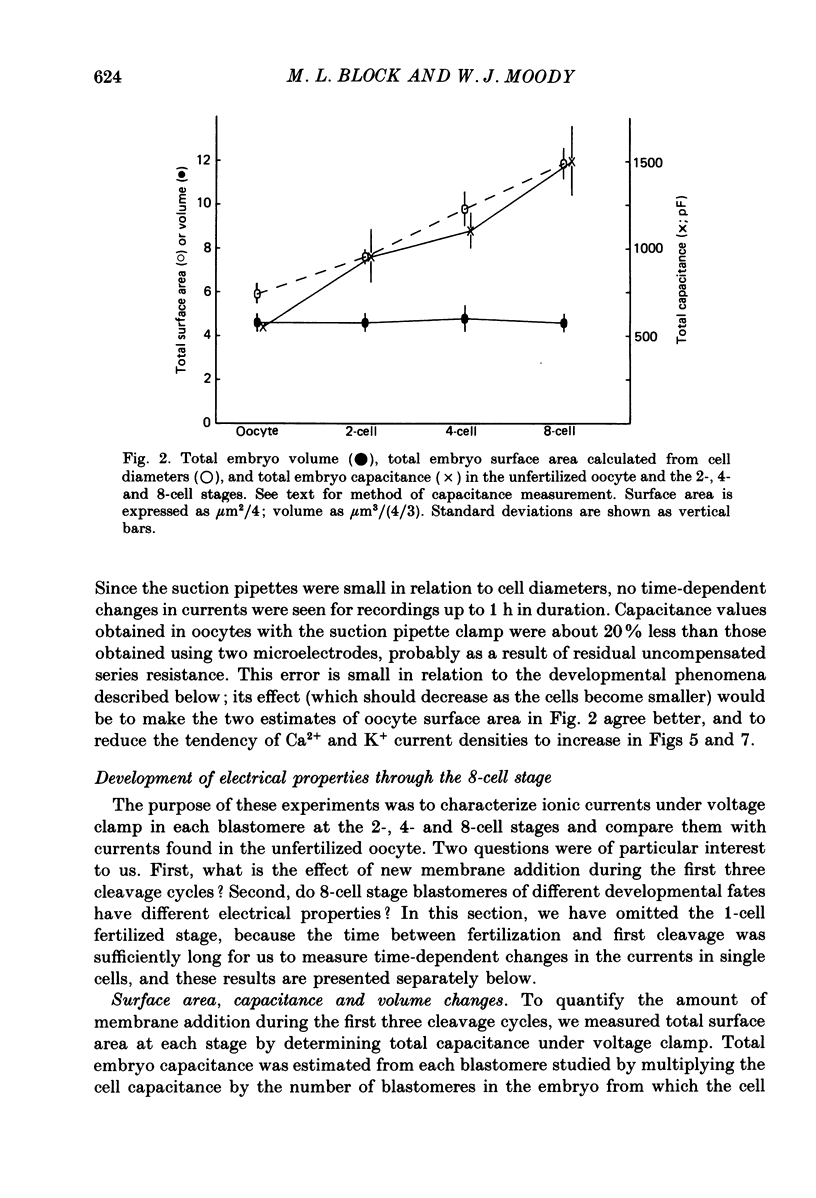
PDF



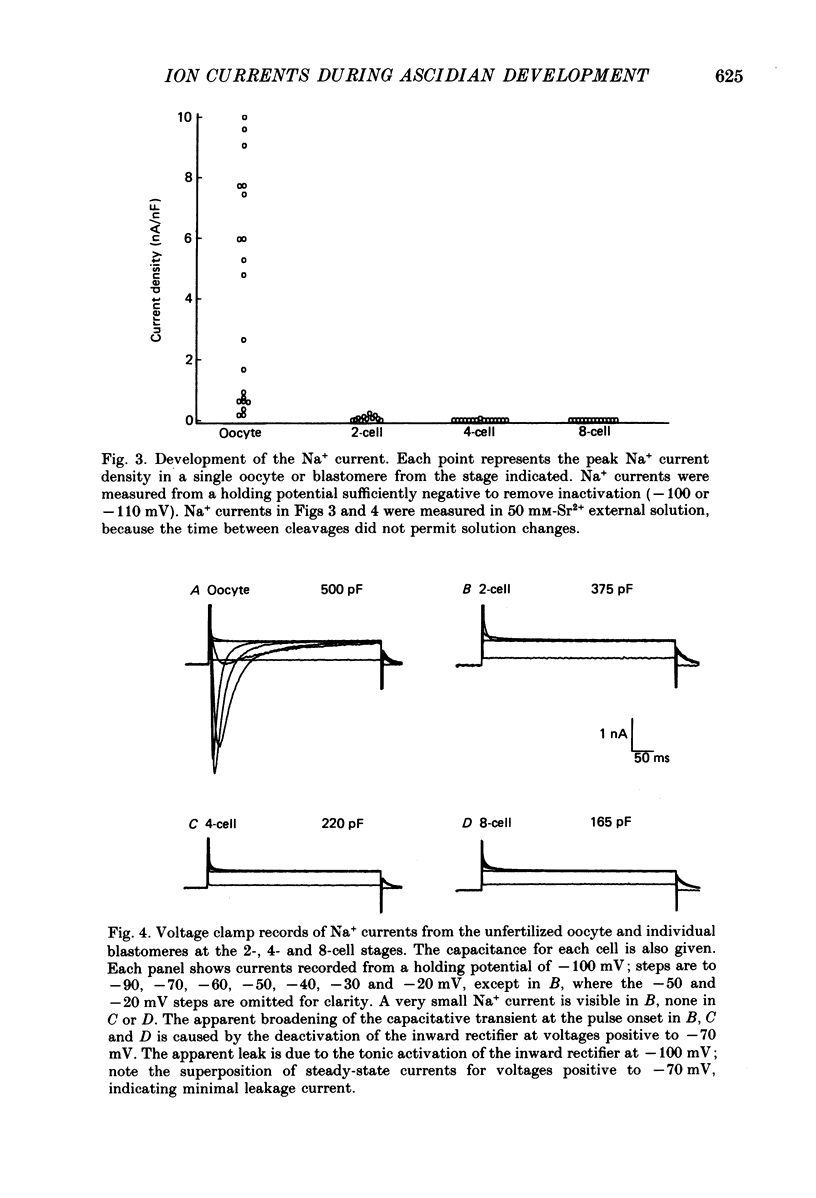

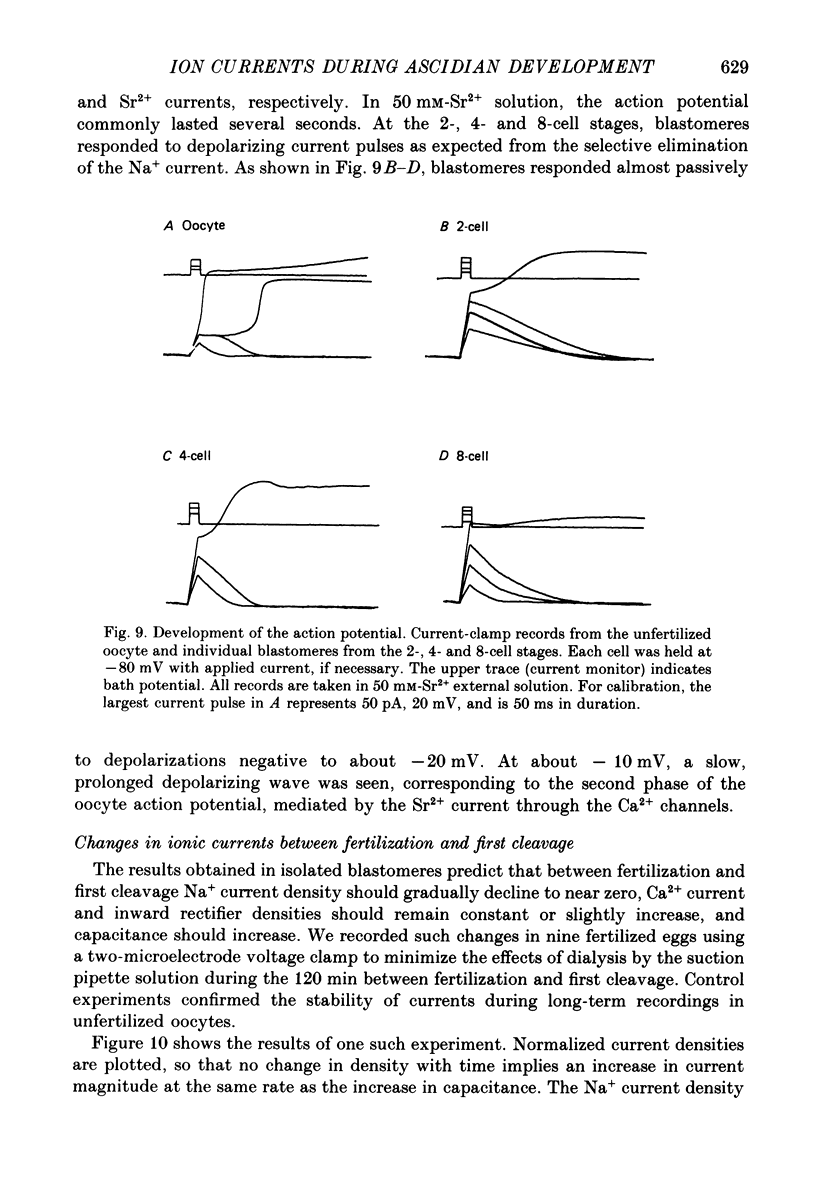




Selected References
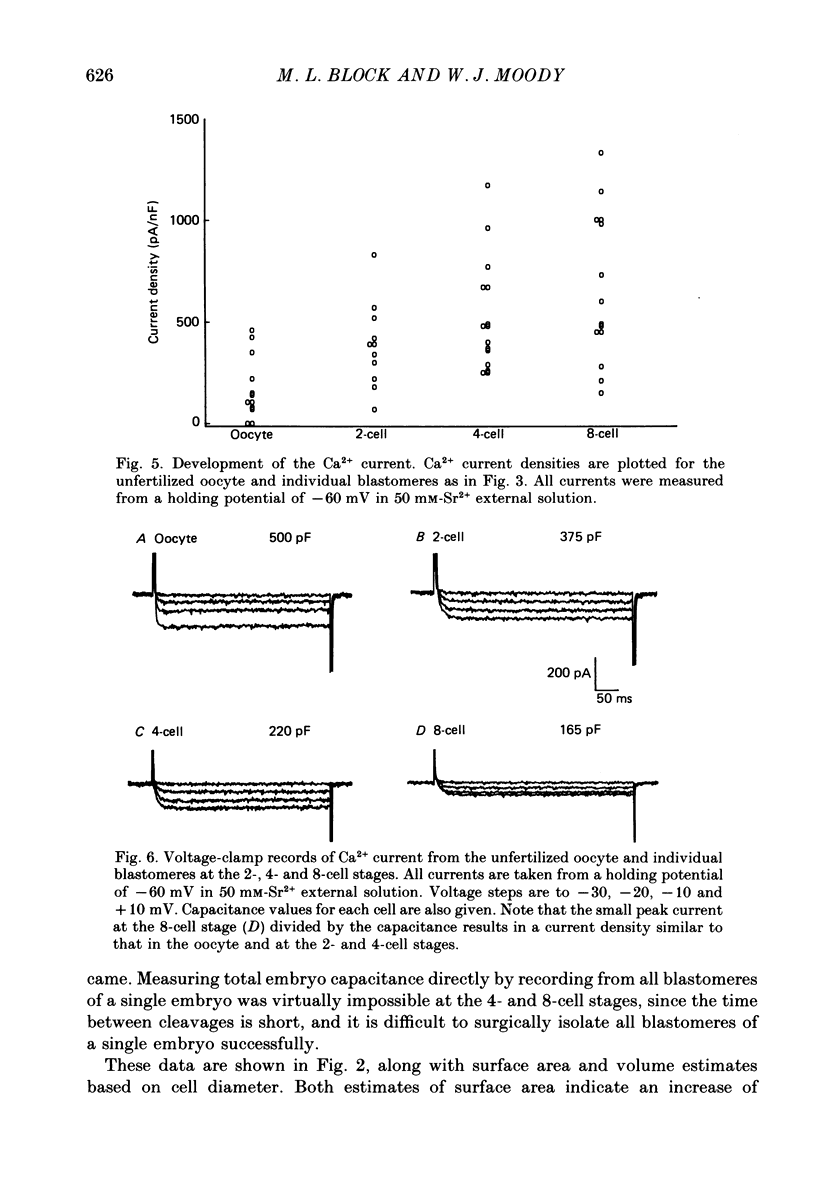
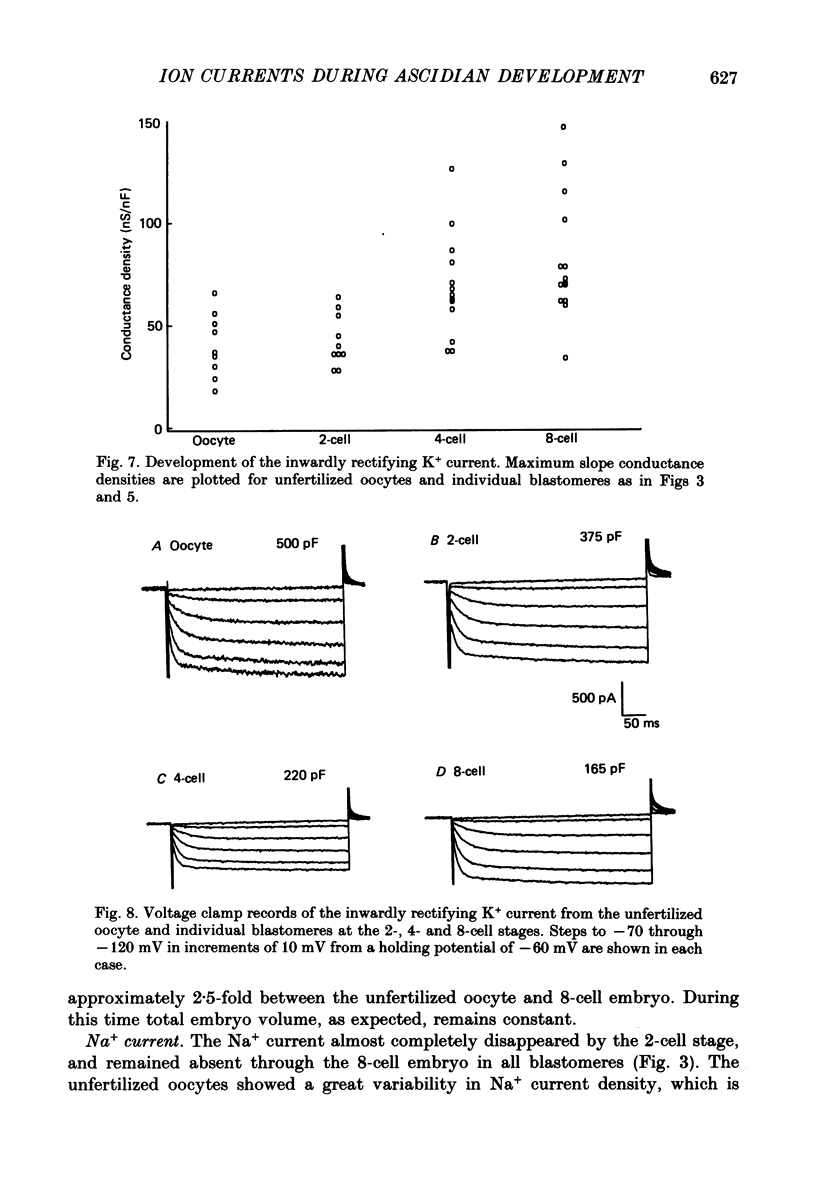
These references are in PubMed. This may not be the complete list of references from this article.
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
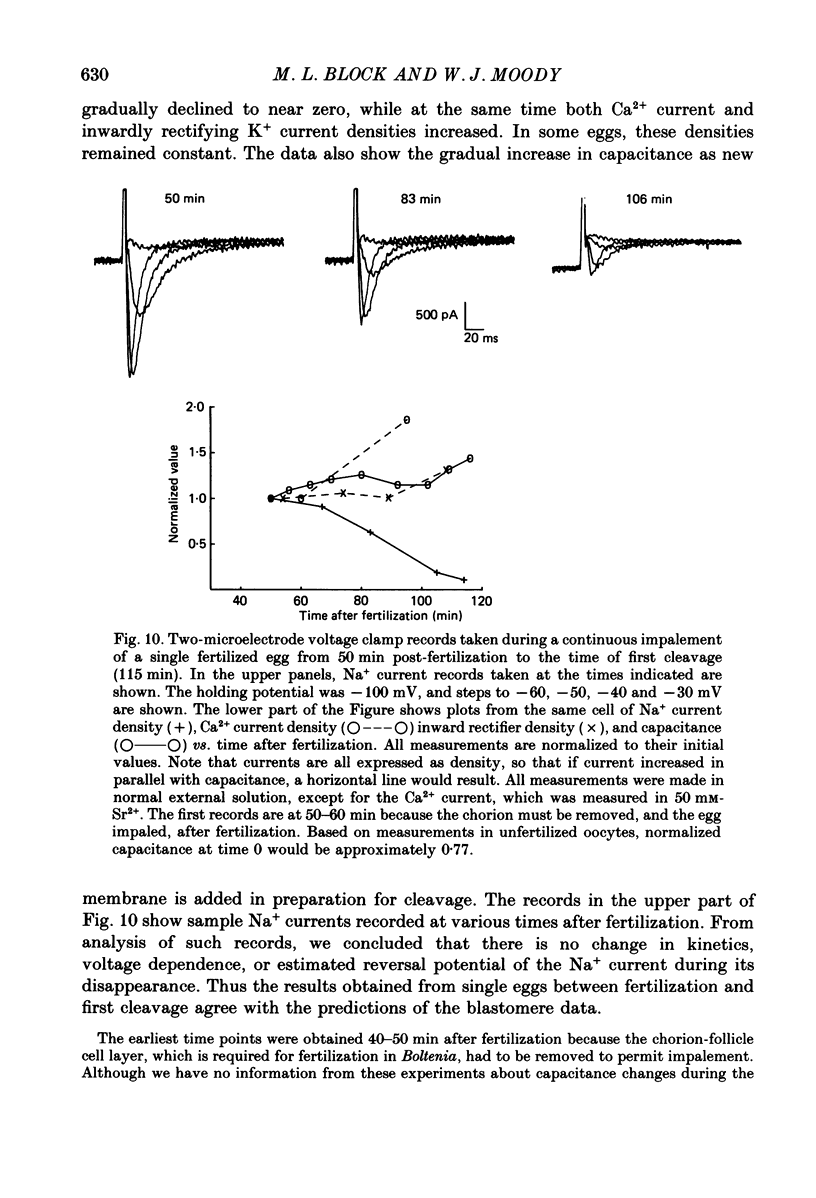
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano T., Takahashi K. Comparison of properties of calcium channels between the differentiated 1-cell embryo and the egg cell of ascidians. J Physiol. 1984 Feb;347:327–344. doi: 10.1113/jphysiol.1984.sp015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Takahashi K., Yamashita N. Determination of excitability types in blastomeres of the cleavage-arrested but differentiated embryos of an ascidian. J Physiol. 1984 Feb;347:301–325. doi: 10.1113/jphysiol.1984.sp015067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A., Guerrier P. Localization of electrical excitability in the early embryo of Dentalium. Dev Biol. 1981 Apr 30;83(2):370–373. doi: 10.1016/0012-1606(81)90483-8. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Calcium ionophore polarizes ooplasmic segregation in ascidian eggs. Science. 1982 Apr 30;216(4545):545–547. doi: 10.1126/science.6803360. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R., Meier S. A yellow crescent cytoskeletal domain in ascidian eggs and its role in early development. Dev Biol. 1983 Mar;96(1):125–143. doi: 10.1016/0012-1606(83)90317-2. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meedel T. H., Whittaker J. R. Development of translationally active mRNA for larval muscle acetylcholinesterase during ascidian embryogenesis. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4761–4765. doi: 10.1073/pnas.80.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J., Bosma M. M. Hormone-induced loss of surface membrane during maturation of starfish oocytes: differential effects on potassium and calcium channels. Dev Biol. 1985 Dec;112(2):396–404. doi: 10.1016/0012-1606(85)90412-9. [DOI] [PubMed] [Google Scholar]
- Moody W. J. The development of calcium and potassium currents during oogenesis in the starfish, Leptasterias hexactis. Dev Biol. 1985 Dec;112(2):405–413. doi: 10.1016/0012-1606(85)90413-0. [DOI] [PubMed] [Google Scholar]
- Nishida H., Satoh N. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. I. Up to the eight-cell stage. Dev Biol. 1983 Oct;99(2):382–394. doi: 10.1016/0012-1606(83)90288-9. [DOI] [PubMed] [Google Scholar]
- Nishida H., Satoh N. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. II. The 16- and 32-cell stages. Dev Biol. 1985 Aug;110(2):440–454. doi: 10.1016/0012-1606(85)90102-2. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolani G., Patricolo E., Mansueto C. Trypsin-induced cell surface changes in ascidian embryonic cells: regulation of differentiation of a tissue-specific protein. Exp Cell Res. 1979 Aug;122(1):137–147. doi: 10.1016/0014-4827(79)90568-8. [DOI] [PubMed] [Google Scholar]
- Satoh N., Deno T. Periodic appearance and disappearance of microvilli associated with cleavage cycles in the egg of the ascidian, Halocynthia roretzi. Dev Biol. 1984 Apr;102(2):488–492. doi: 10.1016/0012-1606(84)90214-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Miyazaki S. I., Kidokoro Y. Development of excitability in embryonic muscle cell membranes in certain tunicates. Science. 1971 Jan 29;171(3969):415–418. doi: 10.1126/science.171.3969.415. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Development of sodium, calcium and potassium channels in the cleavage-arrested embryo of an ascidian. J Physiol. 1981 Jun;315:515–529. doi: 10.1113/jphysiol.1981.sp013761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Knier J. Spontaneous action potentials and resting potential shifts in fertilized eggs of the tunicate Clavelina. Dev Biol. 1983 Sep;99(1):121–131. doi: 10.1016/0012-1606(83)90259-2. [DOI] [PubMed] [Google Scholar]
- Whittaker J. R. Muscle lineage cytoplasm can change the developmental expression in epidermal lineage cells of ascidian embryos. Dev Biol. 1982 Oct;93(2):463–470. doi: 10.1016/0012-1606(82)90134-8. [DOI] [PubMed] [Google Scholar]
- Whittaker J. R., Ortolani G., Farinella-Ferruzza N. Autonomy of acetylcholinesterase differentiation in muscle lineage cells of ascidian embryos. Dev Biol. 1977 Jan;55(1):196–200. doi: 10.1016/0012-1606(77)90331-1. [DOI] [PubMed] [Google Scholar]


