Abstract
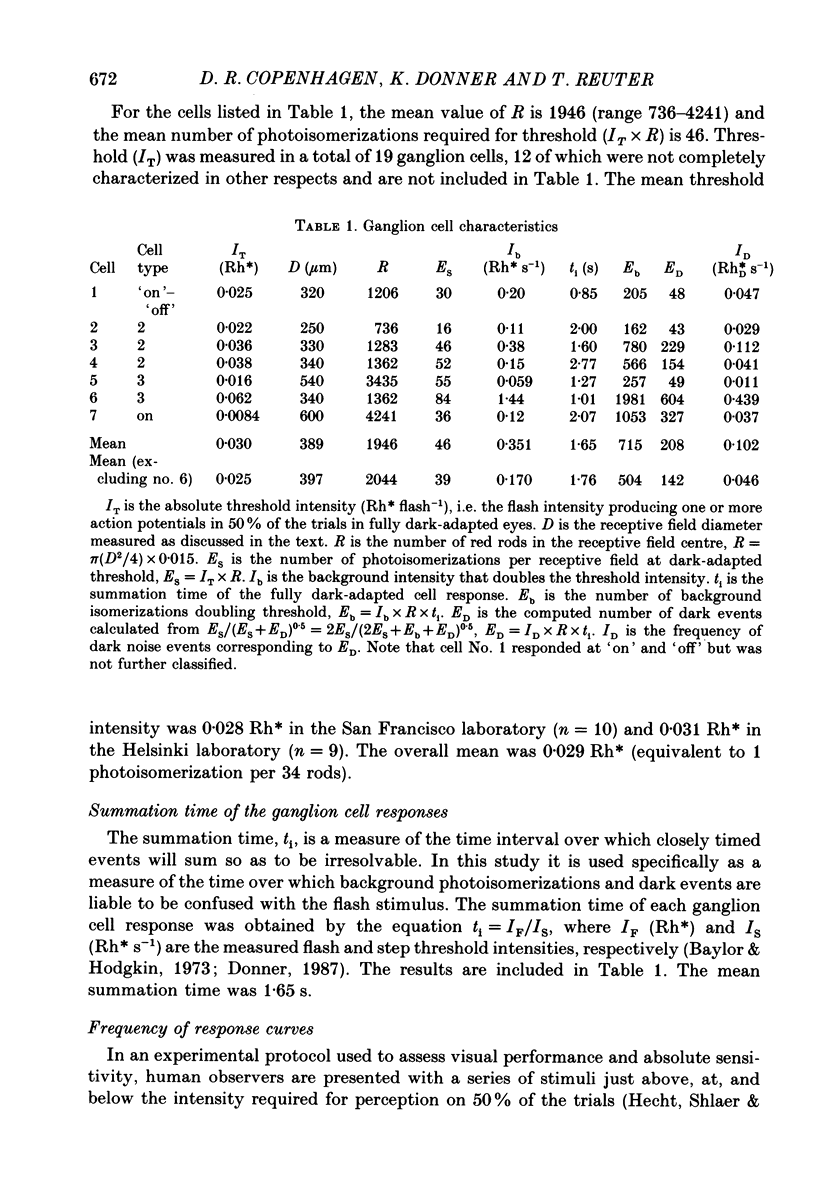
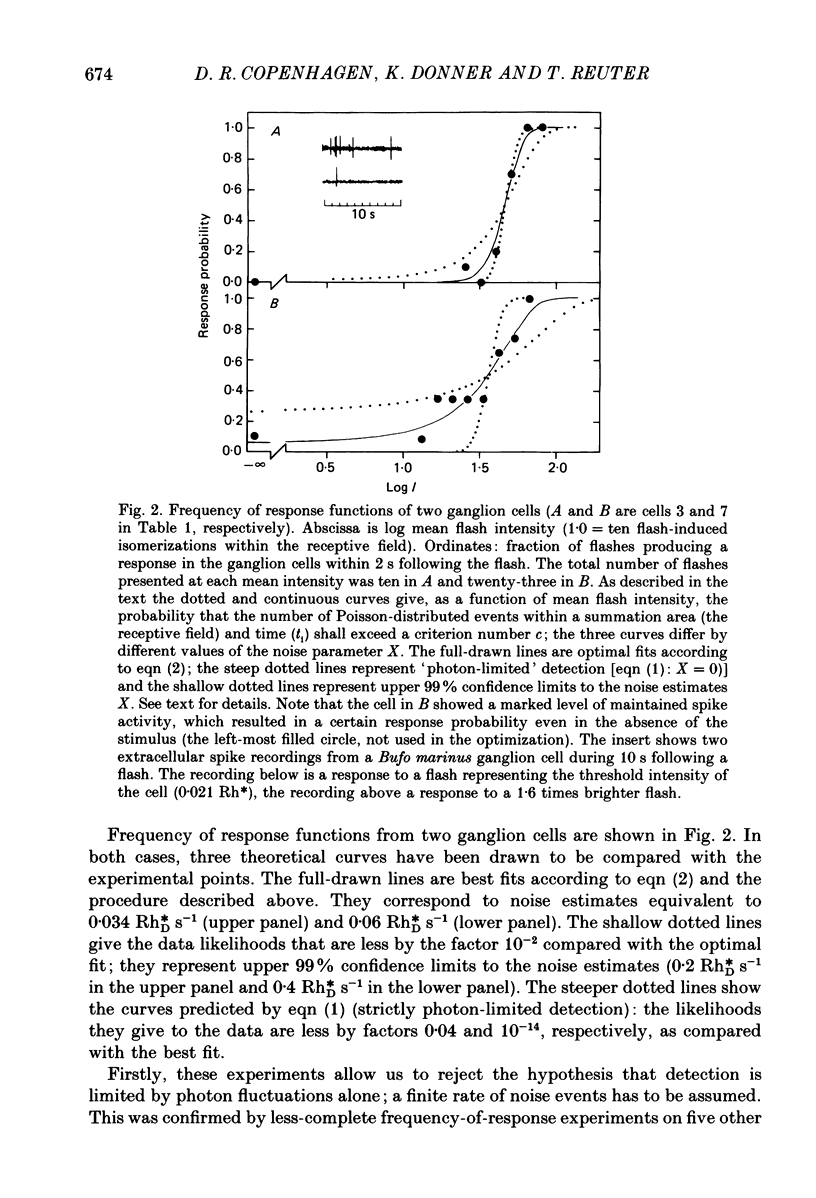
1. The performance of ganglion cells in detecting flashes of light near the absolute threshold was studied in an isolated eye-cup preparation of toad retina. Retinal ganglion cells, through which all visual information from the rods must flow to the brain, are in a key position for evaluating the still unproven hypothesis that the absolute light sensitivity is limited by rod noise (Barlow, 1956). 2. The dark-adapted threshold intensity for these cells, which were selected on the basis of their high sensitivity, averaged 0.029 Rh* flash-1 (range 0.008-0.062), where Rh* signifies one photoisomerization per rod. On average, 46 photoisomerizations were needed per receptive field per flash to evoke a threshold response (range 16-84). 3. In the threshold region, frequency of responses versus mean flash intensity was determined. Threshold performance could be described by theoretical frequency of response curves, allowing intrinsic noise to be estimated in terms of an equivalent rate of photoisomerization-like (dark) events. In two completely characterized cells the rate of dark events corresponded to 0.03 and 0.06 Rh*DS-1, where Rh*D signifies one dark event per rod. 4. Threshold elevations produced by dim backgrounds were studied. The results of these experiments are consistent with a dark event rate equivalent to 0.046 Rh*DS-1, or 0.037 Rh*DS-1 after correcting for a probable decrease in summation time. 5. The rate of actual dark events (0.028 Rh*DS-1, 20 degrees C) measured in Bufo rods (Baylor, Lamb & Yau, 1980) is close to the equivalent rates determined here. Thus, for the ganglion cells signalling the dimmest lights, the dark events in rods appear to be the most significant intrinsic retinal noise source limiting detection.
Full text
PDF





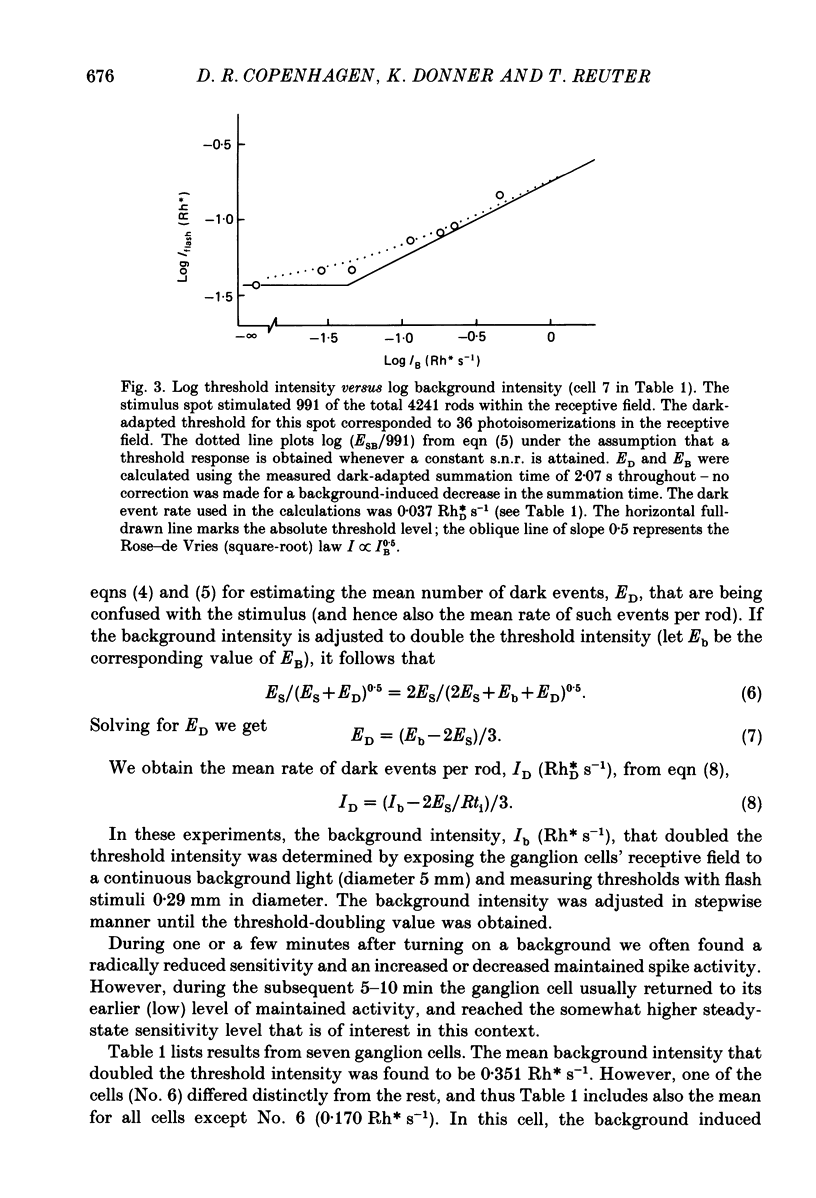




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. An analysis of voltage noise in rod bipolar cells of the dogfish retina. J Physiol. 1982 Nov;332:273–297. doi: 10.1113/jphysiol.1982.sp014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Dark noise in retinal bipolar cells and stability of rhodopsin in rods. Nature. 1977 Nov 3;270(5632):69–71. doi: 10.1038/270069a0. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Retinal noise and absolute threshold. J Opt Soc Am. 1956 Aug;46(8):634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
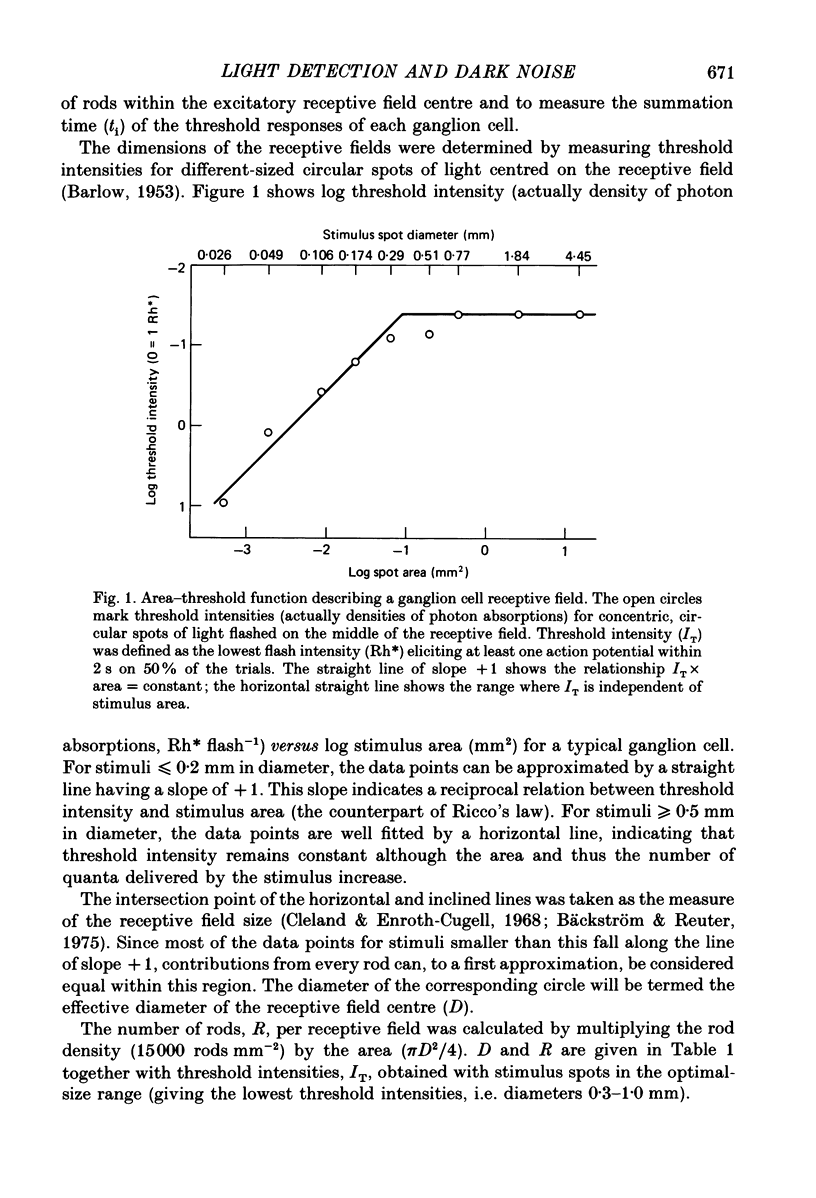
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. D. The molar absorbance coefficient of rhodopsin. Vision Res. 1971 Aug;11(8):841–848. doi: 10.1016/0042-6989(71)90006-x. [DOI] [PubMed] [Google Scholar]
- Brown K. T., Flaming D. G. Effects of Ba2+ upon the dark-adapted intensity-response curve of toad rods. Vision Res. 1979;19(4):395–398. doi: 10.1016/0042-6989(79)90102-0. [DOI] [PubMed] [Google Scholar]
- Bäckström A. C., Hemilä S., Reuter T. Directional selectivity and colour coding in the frog retina. Med Biol. 1978 Apr;56(2):72–83. [PubMed] [Google Scholar]
- Bäckström A. C., Reuter T. Receptive field organization of ganglion cells in the frog retina: contributions from cones, green rods and red rods. J Physiol. 1975 Mar;246(1):79–107. doi: 10.1113/jphysiol.1975.sp010881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. The dark-adaptation of single units in the frog's retina and its relation to the regeneration of rhodopsin. Vision Res. 1965 Dec;5(11):615–632. doi: 10.1016/0042-6989(65)90035-0. [DOI] [PubMed] [Google Scholar]
- Ewert J. P., Hock F. Movement-sensitive neurones in the toad's retina. Exp Brain Res. 1972;16(1):41–59. doi: 10.1007/BF00233373. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman L. J., Levine M. W. Statistics of the maintained discharge of cat retinal ganglion cells. J Physiol. 1983 Jun;339:475–494. doi: 10.1113/jphysiol.1983.sp014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G. H. Photoreceptor coupling in retina of the toad, Bufo marinus. II. Physiology. J Neurophysiol. 1979 Jan;42(1 Pt 1):311–328. doi: 10.1152/jn.1979.42.1.311. [DOI] [PubMed] [Google Scholar]
- Griff E. R., Pinto L. H. Interactions among rods in the isolated retina of Bufo marinus. J Physiol. 1981 May;314:237–254. doi: 10.1113/jphysiol.1981.sp013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., Pirenne M. H. ENERGY, QUANTA, AND VISION. J Gen Physiol. 1942 Jul 20;25(6):819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. T., Stell W. K., Lightfoot D. O. Rod and cone inputs to bipolar cells in goldfish retina. J Comp Neurol. 1980 Jun;191(3):315–335. doi: 10.1002/cne.901910302. [DOI] [PubMed] [Google Scholar]
- Leeper H. F., Normann R. A., Copenhagen D. R. Evidence for passive electrotonic interactions in red rods of toad retina. Nature. 1978 Sep 21;275(5677):234–236. doi: 10.1038/275234b0. [DOI] [PubMed] [Google Scholar]
- Lillywhite P. G. Multiplicative intrinsic noise and the limits to visual performance. Vision Res. 1981;21(2):291–296. doi: 10.1016/0042-6989(81)90123-1. [DOI] [PubMed] [Google Scholar]
- Matthews G. Dark noise in the outer segment membrane current of green rod photoreceptors from toad retina. J Physiol. 1984 Apr;349:607–618. doi: 10.1113/jphysiol.1984.sp015176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE A. The sensitivity performance of the human eye on an absolute scale. J Opt Soc Am. 1948 Feb;38(2):196–208. doi: 10.1364/josa.38.000196. [DOI] [PubMed] [Google Scholar]
- Reuter T. E., White R. H., Wald G. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J Gen Physiol. 1971 Oct;58(4):351–371. doi: 10.1085/jgp.58.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter T., Donner K., Copenhagen D. R. Does the random distribution of discrete photoreceptor events limit the sensitivity of the retina? Neurosci Res Suppl. 1986;4:S163–S180. doi: 10.1016/0168-0102(86)90081-7. [DOI] [PubMed] [Google Scholar]


