Abstract
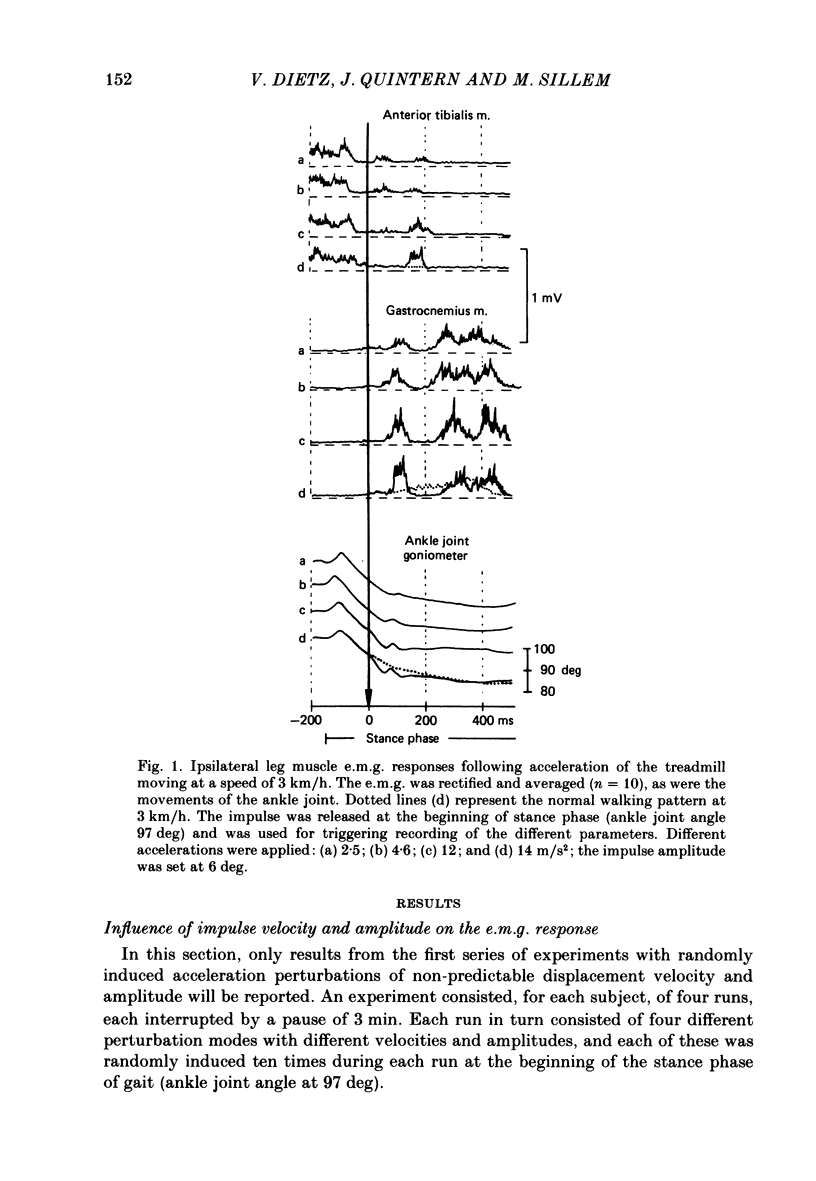
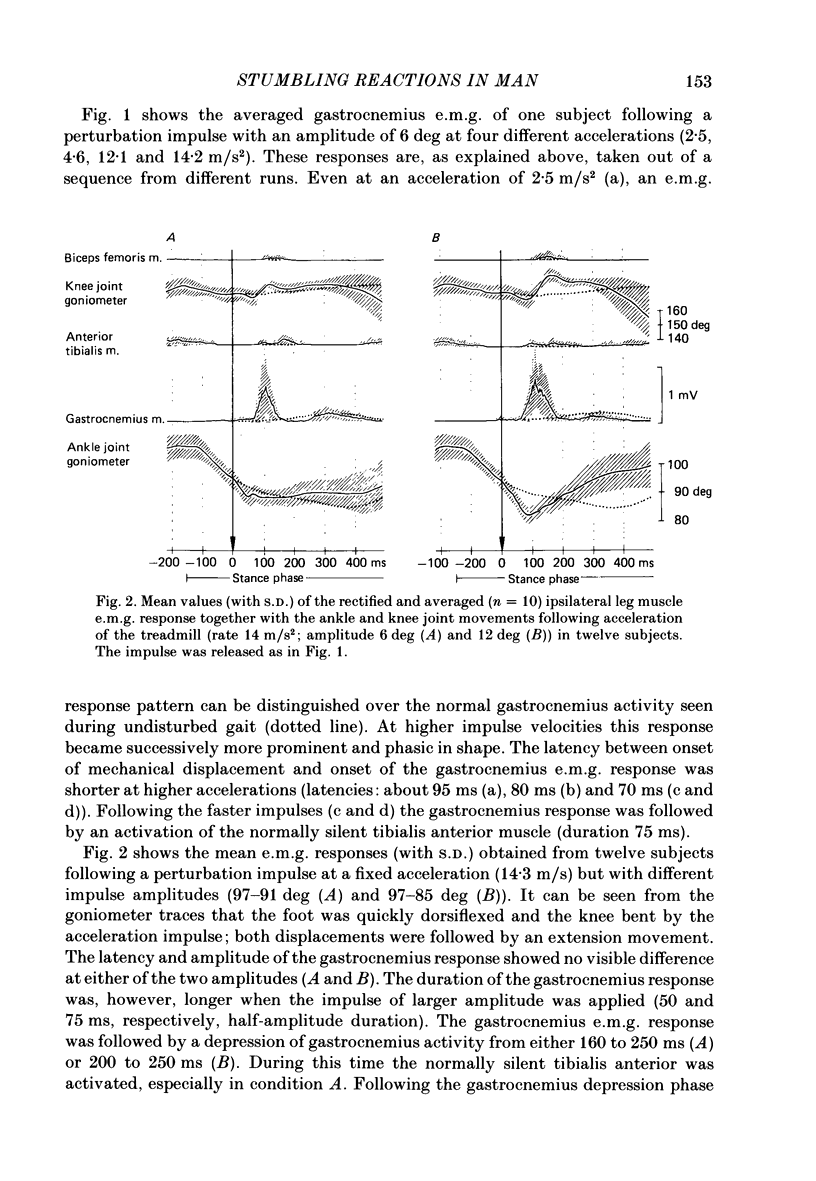
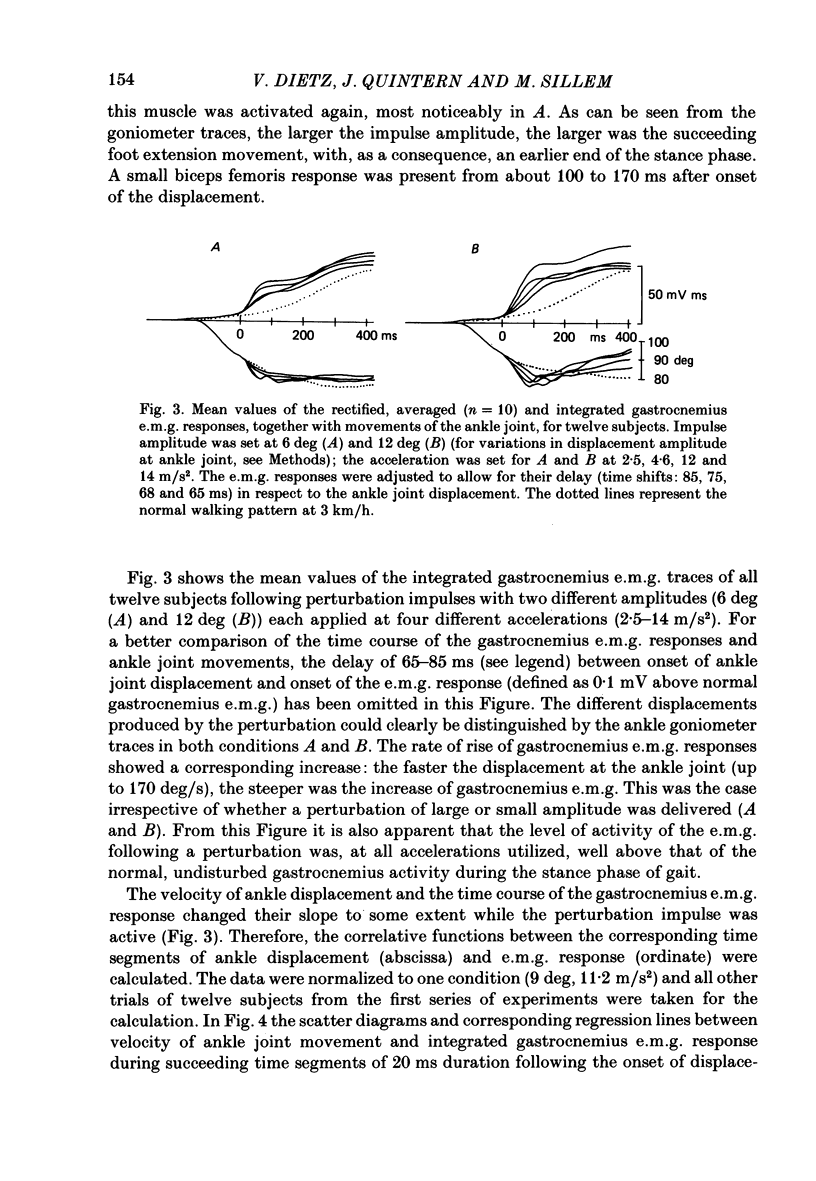
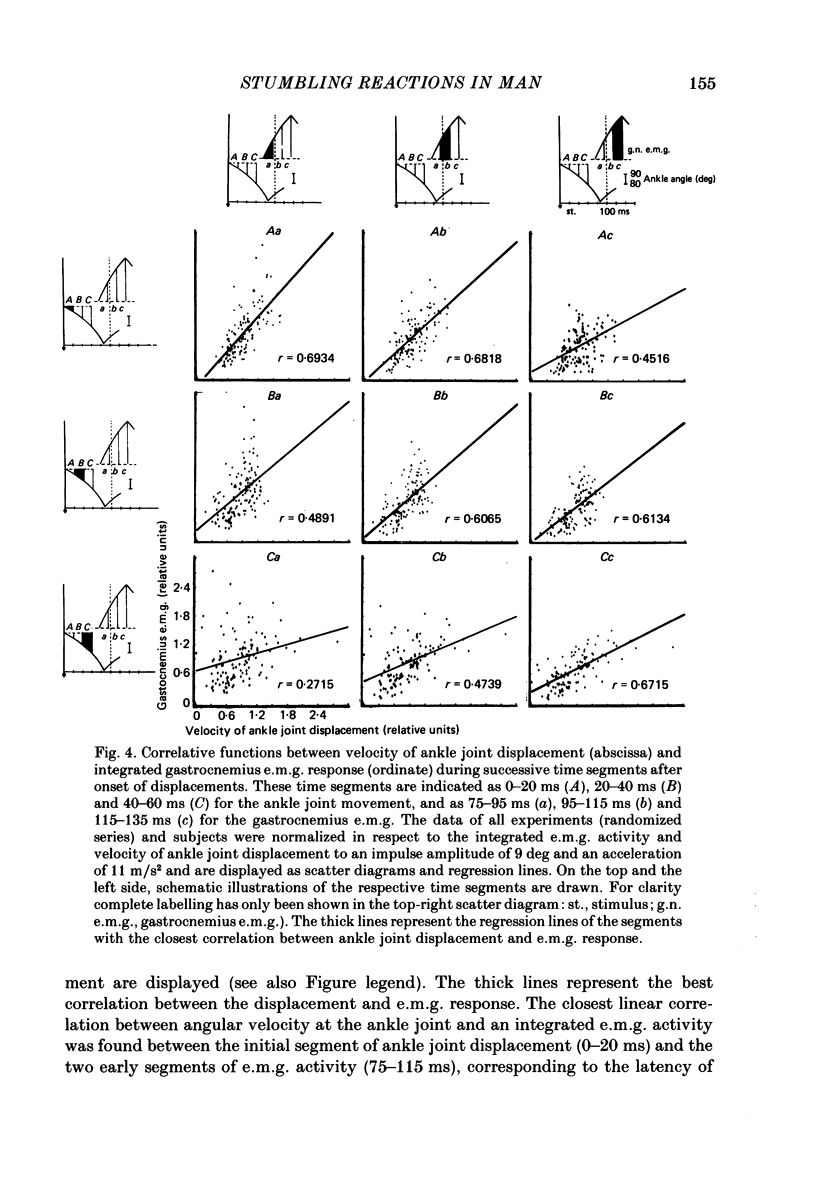
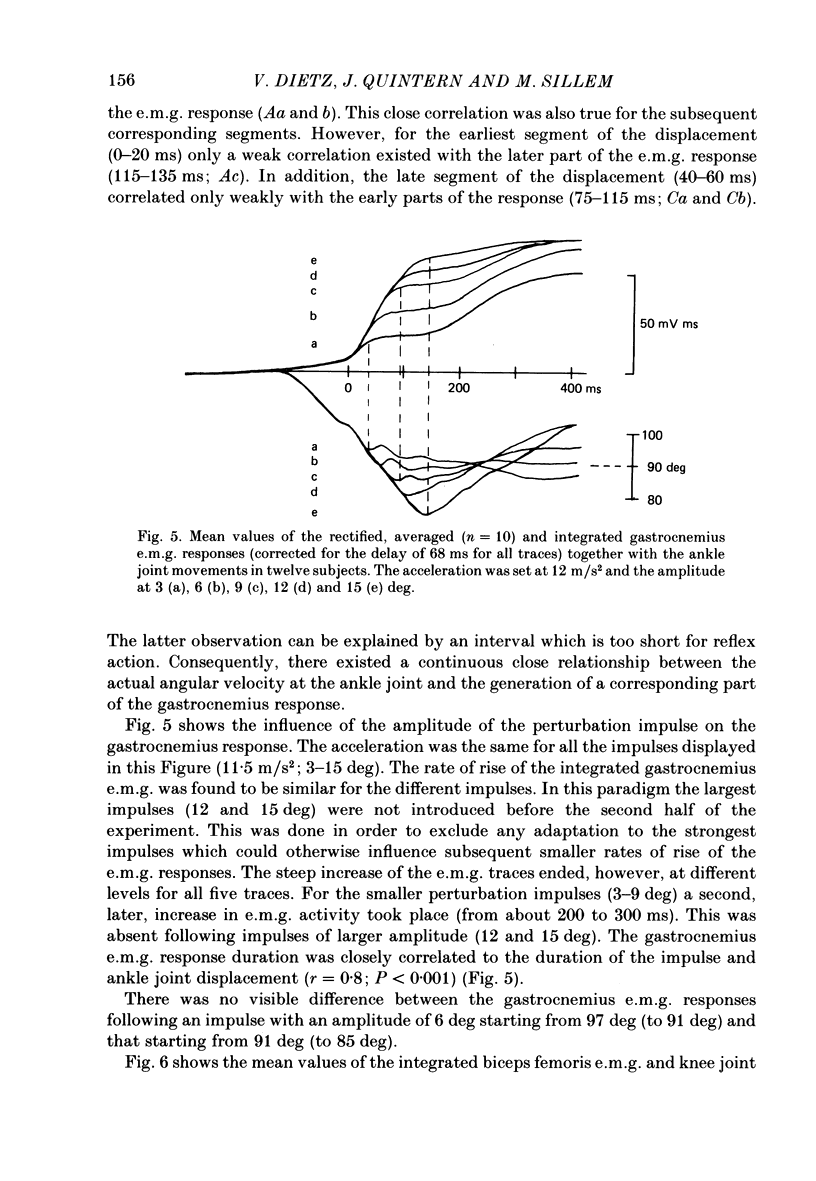
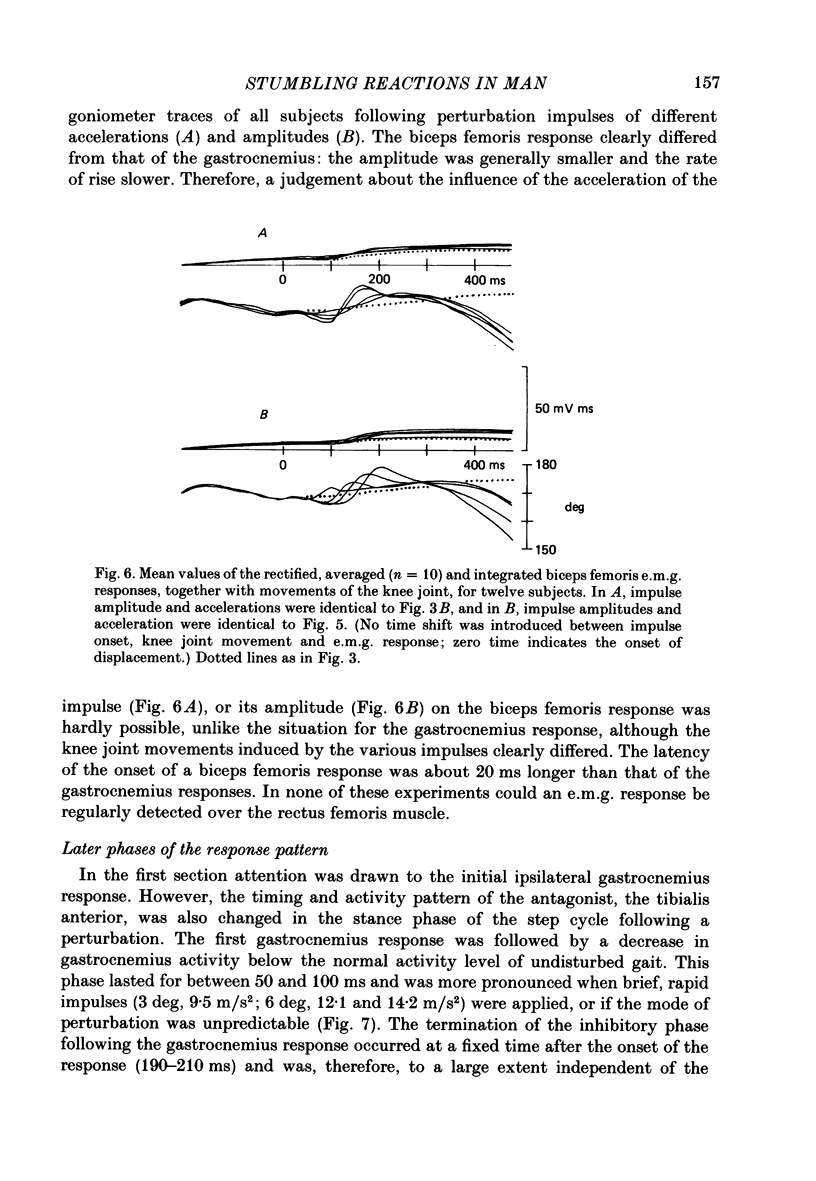
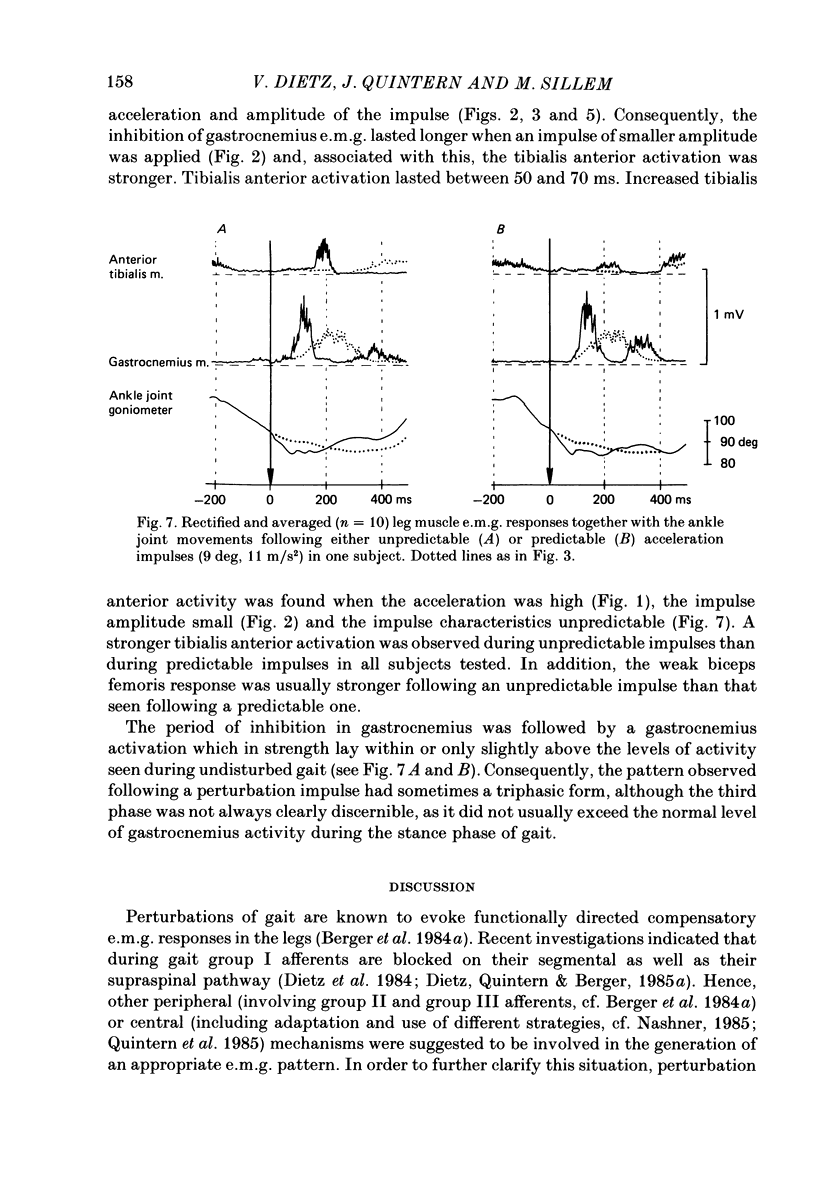
1. Electromyogram (e.m.g.) responses of the leg musculature and the corresponding joint movements were studied following a perturbation of the limb during walking on a treadmill, produced by a randomly timed treadmill acceleration impulse, either predictable, or unpredictable in its amplitude and rate of acceleration. 2. The rate of rise of ipsilateral gastrocnemius e.m.g. response following a perturbation was dependent on the rate of treadmill acceleration. For a given acceleration rate the amplitude of the e.m.g. response and the timing of its peak was dependent on the amplitude of the impulse and the rate of rise of the gastrocnemius response was the same for impulses of both small and large amplitude. The onset latency was shorter (65 ms) for high accelerations and longer (85 ms) for lower ones. 3. The amplitude of the ipsilateral biceps femoris response was much smaller than the gastrocnemius response but was larger following unpredictable than predictable impulses. 4. The initial gastrocnemius response was followed by a tibialis anterior activation associated with a gastrocnemius depression and sometimes with a second, weak gastrocnemius activation. The gastrocnemius depression ended within a fixed time range relative to the onset of the response. The tibialis anterior activation was most pronounced when unpredictable impulses with high acceleration but a small amplitude were induced. 5. It is concluded that generation of the first gastrocnemius response is obviously under continuous control by muscle proprioceptive information and can be best described in terms of a stretch reflex response. It is suggested that, on the evidence of the diphasic or triphasic e.m.g. pattern, a close interaction occurs between a central programme and muscle proprioceptive input in order to generate the appropriate e.m.g. pattern. 6. On the basis of earlier work (Berger, Dietz & Quintern, 1984a) and on the present results it is suggested that the e.m.g. responses may be mediated mainly by muscle proprioceptive input from group II afferents. This input is modulated and processed by spinal interneuronal circuits, closely connected with spinal locomotor centres. The mode of processing depends on various factors, such as the predictability of the nature of the impulse.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allum J. H., Pfaltz C. R. Visual and vestibular contributions to pitch sway stabilization in the ankle muscles of normals and patients with bilateral peripheral vestibular deficits. Exp Brain Res. 1985;58(1):82–94. doi: 10.1007/BF00238956. [DOI] [PubMed] [Google Scholar]
- Berardelli A., Hallett M., Kaufman C., Fine E., Berenberg W., Simon S. R. Stretch reflexes of triceps surae in normal man. J Neurol Neurosurg Psychiatry. 1982 Jun;45(6):513–525. doi: 10.1136/jnnp.45.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A., Rothwell J. C., Day B. L., Kachi T., Marsden C. D. Duration of the first agonist EMG burst in ballistic arm movements. Brain Res. 1984 Jun 18;304(1):183–187. doi: 10.1016/0006-8993(84)90879-5. [DOI] [PubMed] [Google Scholar]
- Berardelli A., Sabra A. F., Hallett M., Berenberg W., Simon S. R. Stretch reflexes of triceps surae in patients with upper motor neuron syndromes. J Neurol Neurosurg Psychiatry. 1983 Jan;46(1):54–60. doi: 10.1136/jnnp.46.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W., Dietz V., Quintern J. Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. J Physiol. 1984 Dec;357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W., Horstmann G., Dietz V. Tension development and muscle activation in the leg during gait in spastic hemiparesis: independence of muscle hypertonia and exaggerated stretch reflexes. J Neurol Neurosurg Psychiatry. 1984 Sep;47(9):1029–1033. doi: 10.1136/jnnp.47.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton K., Lippold O. C., Shahani M., Shahani U. Long-latency spinal reflexes in humans. J Neurophysiol. 1985 Jun;53(6):1604–1618. doi: 10.1152/jn.1985.53.6.1604. [DOI] [PubMed] [Google Scholar]
- Diener H. C., Bootz F., Dichgans J., Bruzek W. Variability of postural "reflexes" in humans. Exp Brain Res. 1983;52(3):423–428. doi: 10.1007/BF00238035. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Afferent control of human stance and gait: evidence for blocking of group I afferents during gait. Exp Brain Res. 1985;61(1):153–163. doi: 10.1007/BF00235630. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci Lett. 1984 Feb 10;44(2):131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W., Schenck E. Cerebral potentials and leg muscle e.m.g. responses associated with stance perturbation. Exp Brain Res. 1985;57(2):348–354. doi: 10.1007/BF00236540. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Stumbling reactions in man: release of a ballistic movement pattern. Brain Res. 1986 Jan 8;362(2):355–357. doi: 10.1016/0006-8993(86)90462-2. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Nashner L. M. Ontogenetic development of postural control in man: adaptation to altered support and visual conditions during stance. J Neurosci. 1982 May;2(5):545–552. doi: 10.1523/JNEUROSCI.02-05-00545.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund H. J., Büdingen H. J. The relationship between speed and amplitude of the fastest voluntary contractions of human arm muscles. Exp Brain Res. 1978 Jan 18;31(1):1–12. doi: 10.1007/BF00235800. [DOI] [PubMed] [Google Scholar]
- Hallett M., Marsden C. D. Ballistic flexion movements of the human thumb. J Physiol. 1979 Sep;294:33–50. doi: 10.1113/jphysiol.1979.sp012913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., Shahani B. T., Young R. R. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1154–1162. doi: 10.1136/jnnp.38.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F. B., Nashner L. M. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986 Jun;55(6):1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Lee R. G., Tatton W. G. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975 Aug;2(3):285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Obeso J. A., Rothwell J. C. The function of the antagonist muscle during fast limb movements in man. J Physiol. 1983 Feb;335:1–13. doi: 10.1113/jphysiol.1983.sp014514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritz K. H., Dietz V. Characteristics of postural instability induced by ischemic blocking of leg afferents. Exp Brain Res. 1980;38(1):117–119. doi: 10.1007/BF00237939. [DOI] [PubMed] [Google Scholar]
- Nashner L. M. Adapting reflexes controlling the human posture. Exp Brain Res. 1976 Aug 27;26(1):59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Nashner L. M., Black F. O., Wall C., 3rd Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982 May;2(5):536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner L. M., Woollacott M., Tuma G. Organization of rapid responses to postural and locomotor-like perturbations of standing man. Exp Brain Res. 1979 Aug 1;36(3):463–476. doi: 10.1007/BF00238516. [DOI] [PubMed] [Google Scholar]
- Quintern J., Berger W., Dietz V. Compensatory reactions to gait perturbations in man: short- and long-term effects of neuronal adaptation. Neurosci Lett. 1985 Dec 18;62(3):371–376. doi: 10.1016/0304-3940(85)90577-4. [DOI] [PubMed] [Google Scholar]


