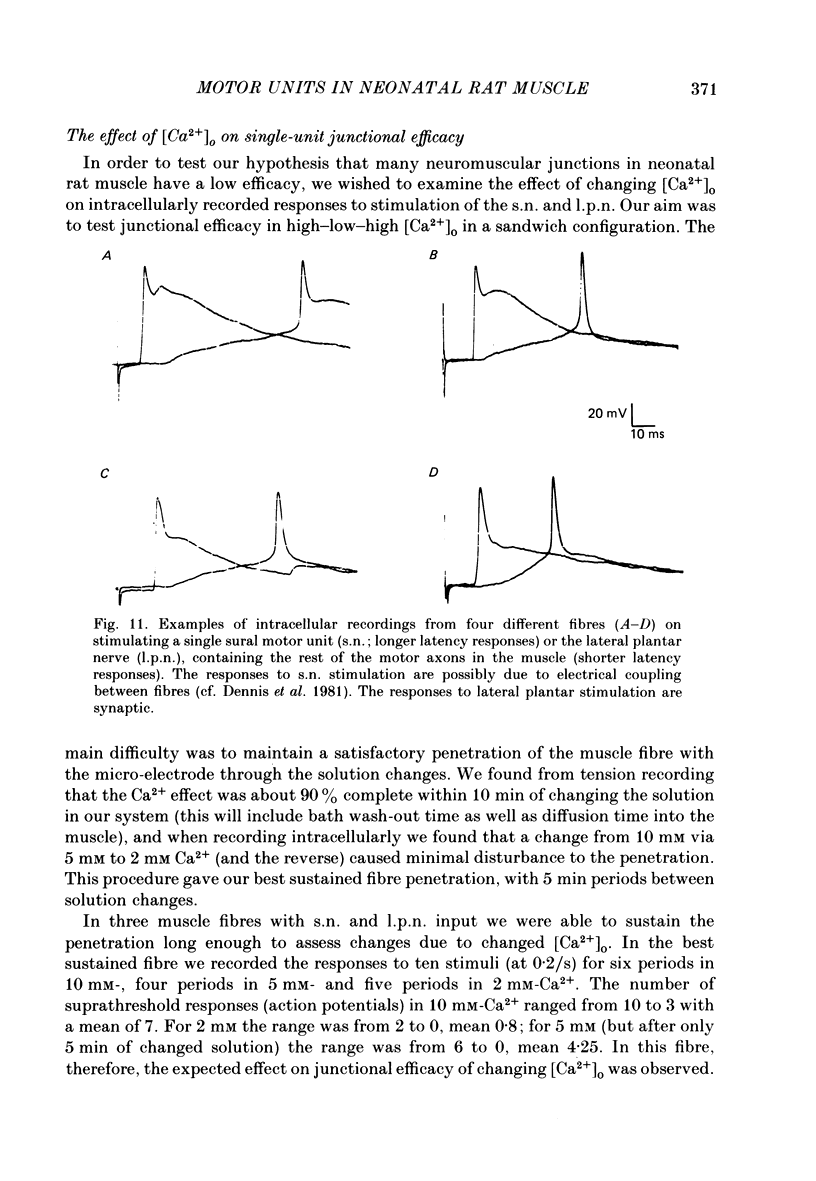
Abstract
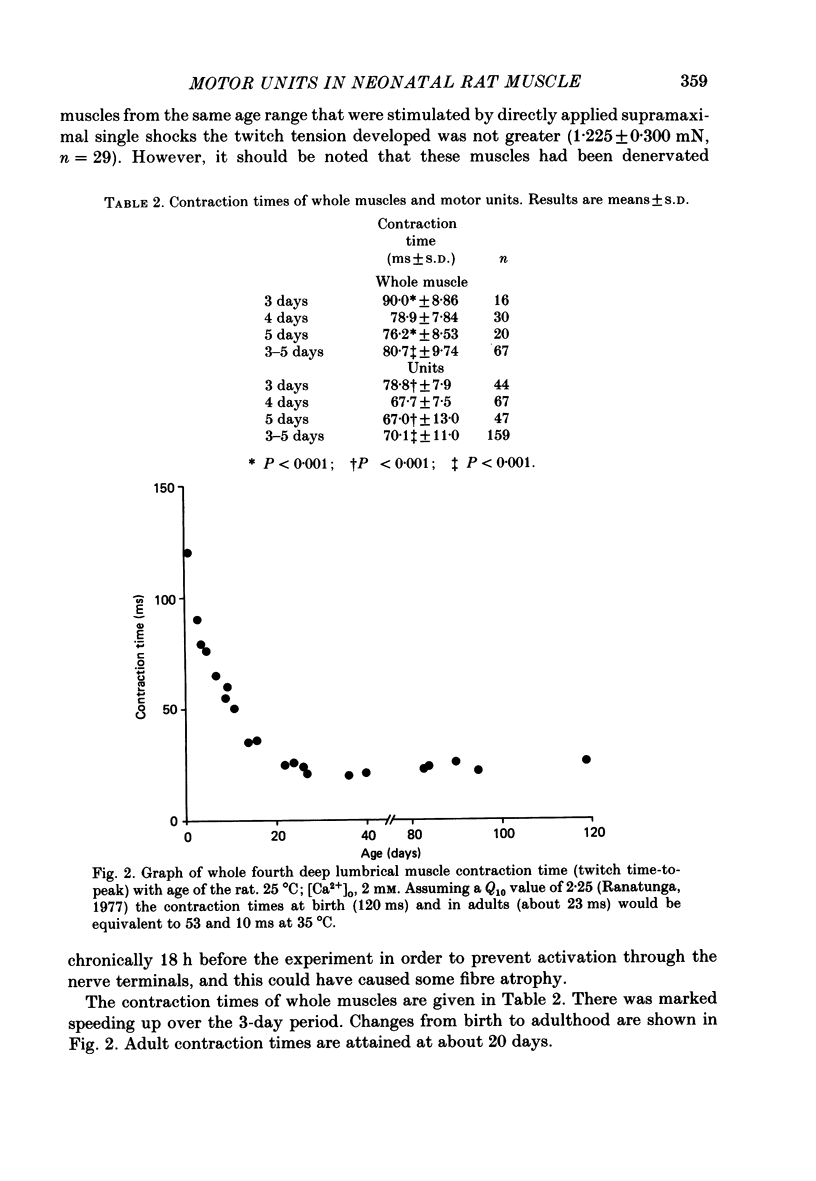
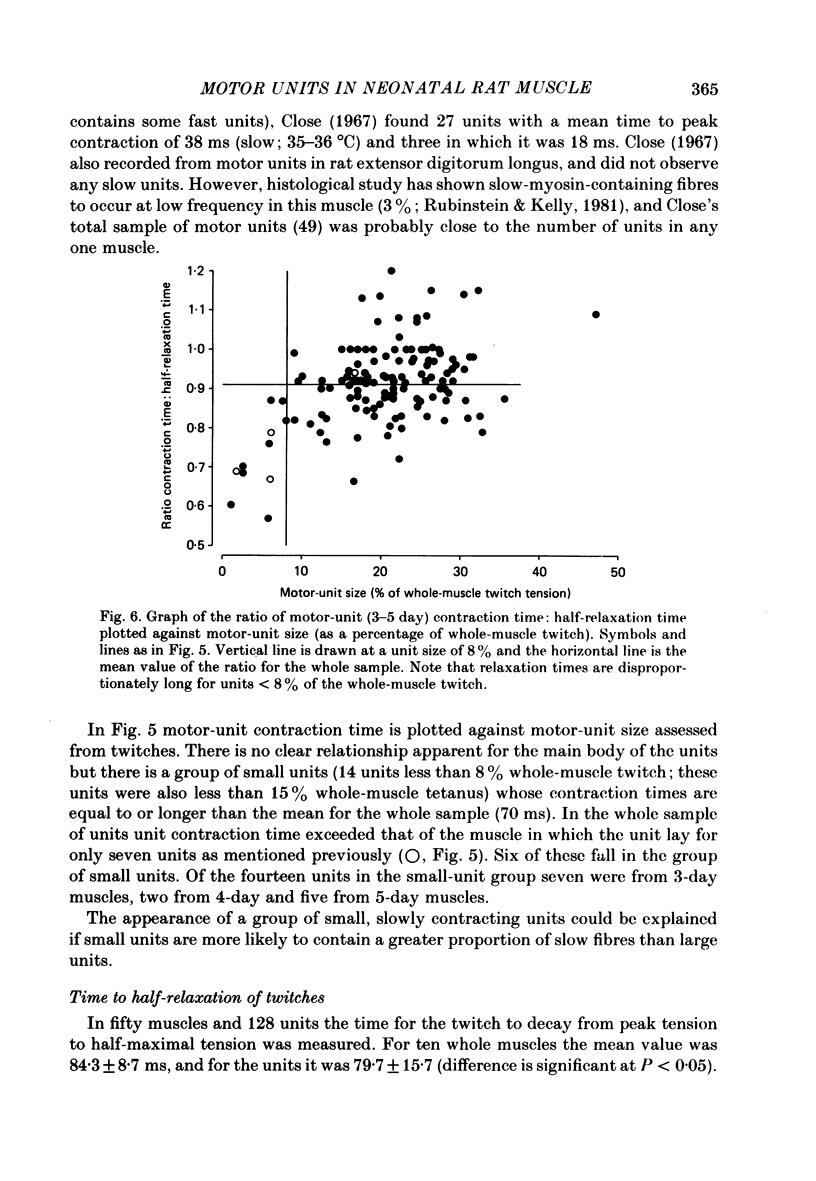
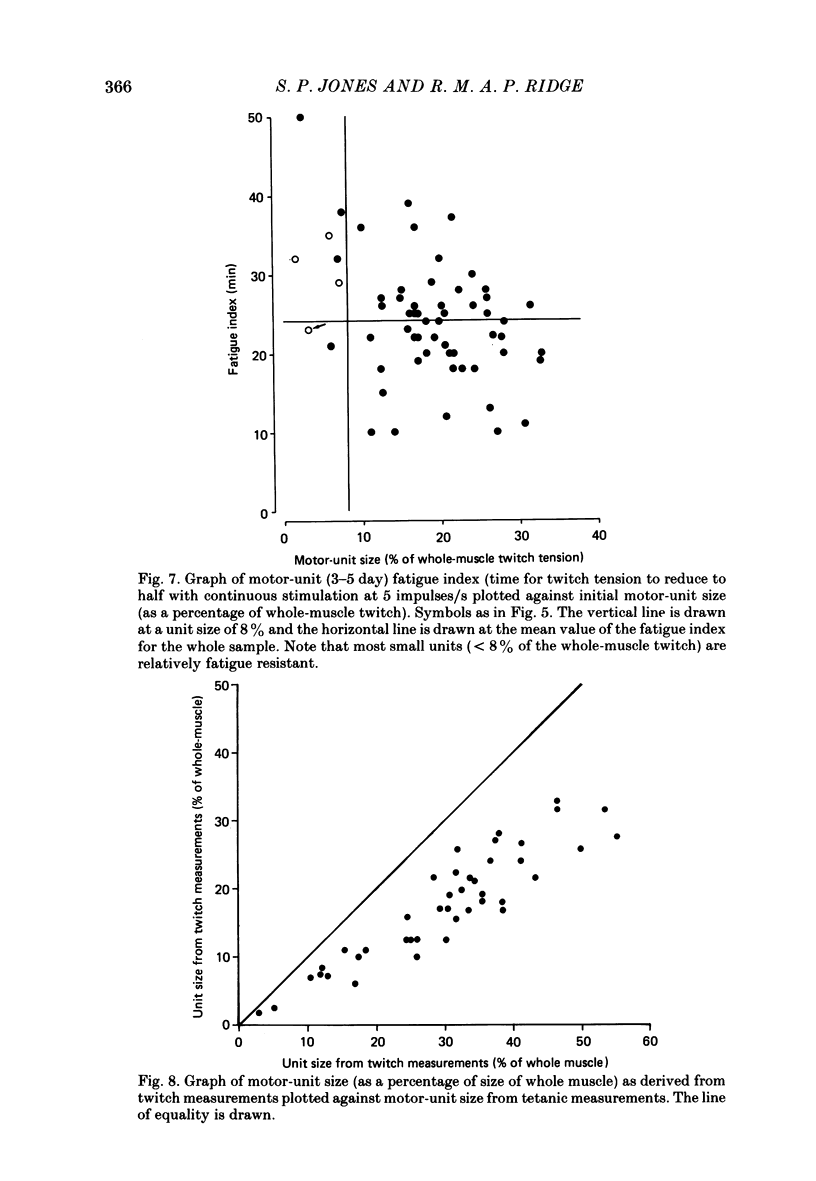
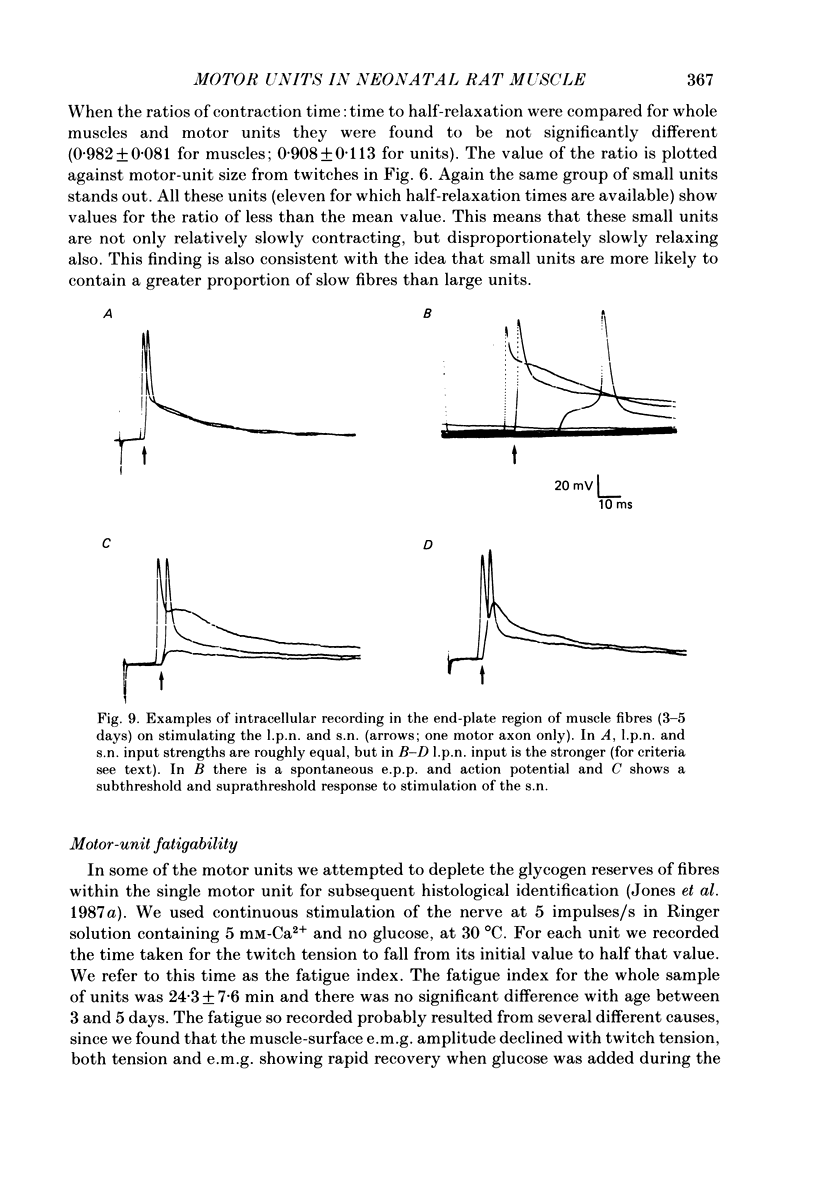
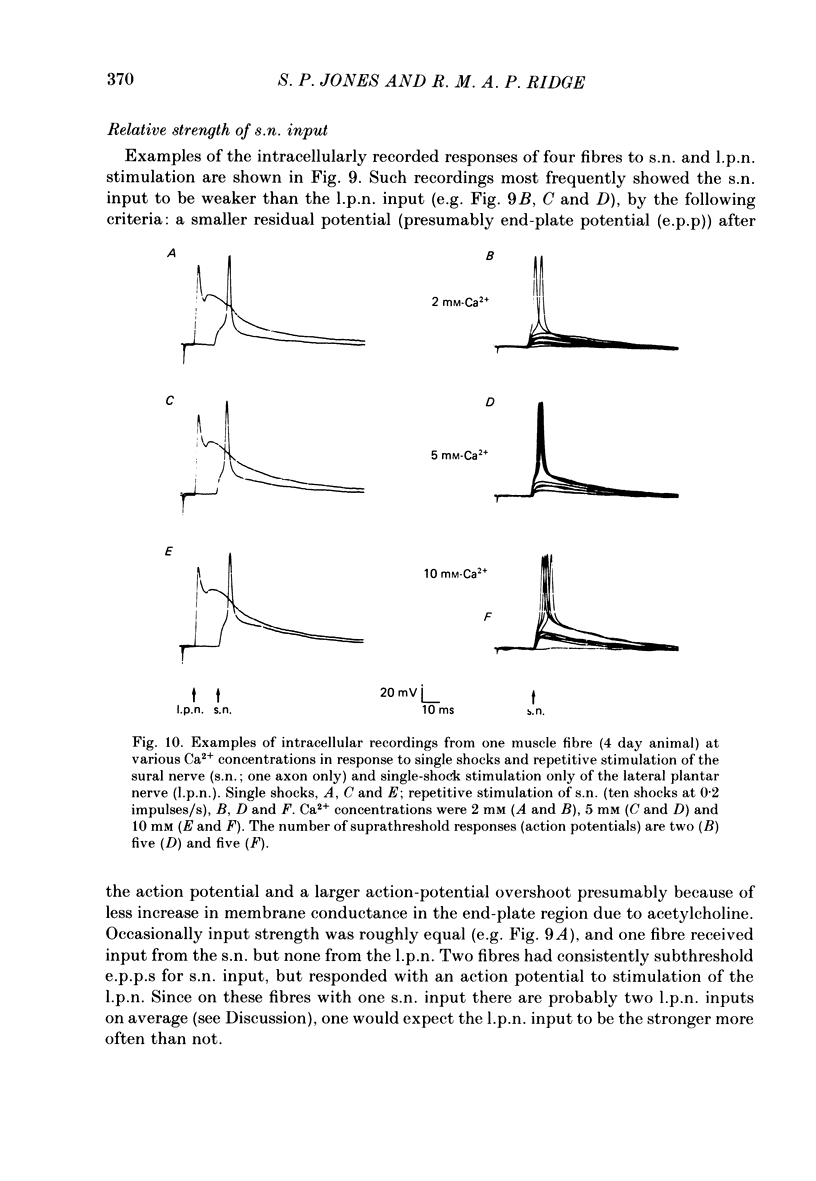
1. Isometric twitch and tetanic tensions were recorded from whole muscles and single motor units in isolated fourth deep lumbrical muscles from neonatal rats (most at 3-5 days old) and from older rats of various ages. 2. Whole-muscle time to peak contraction reduced from about 120 ms at birth to about 20-25 ms at 20 days and older. 3. The number of motor units in the muscle was constant with age (eleven on average) and there was no significant branching of motor axons below the common peroneal nerve branching point in the thigh. 4. In the 3-5 days age range, mean twitch:tetanus ratio for whole muscles was 0.299 and for single units was 0.177. As a consequence, mean motor unit size (as a percentage of whole-muscle tension) was greater for tetani (29.7%) than for twitches (19.9%). This was not the case in muscles from animals 22 days or older. Evidence is given that the cause of this is low junctional efficacy in some neuromuscular junctions in neonatal muscle. Intracellular recordings supported this view. 5. The relationships of motor-unit size to the contraction time, to the ratio of contraction time:half-relaxation time, and to fatigue index are given. There was no indication of clear segregation of motor units into more than one population, but it is concluded that small motor units are more likely to contain a higher proportion of slowly contracting, fatigue-resistant fibres than large units. 6. The level of overlap by axons in the lateral plantar nerve onto muscle fibres in a single sural nerve motor unit was greater in tetani than in twitches. The results indicate that the distribution of weak and strong inputs was not random, but that there was a tendency for one strong input to accompany a number of weak inputs (on average about two) on each muscle fibre. 7. Intracellular recording indicates that about 12% of fibres at 3-5 days may be electrically coupled.
Full text
PDF


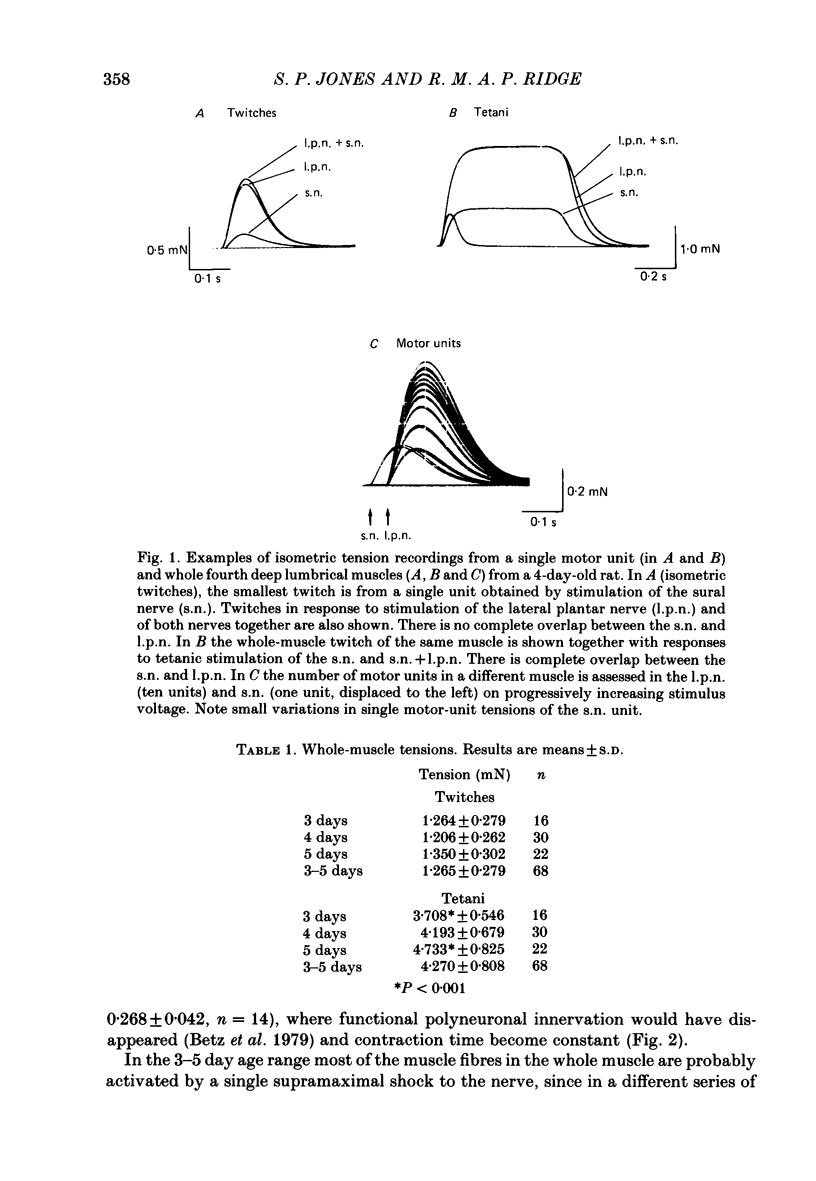



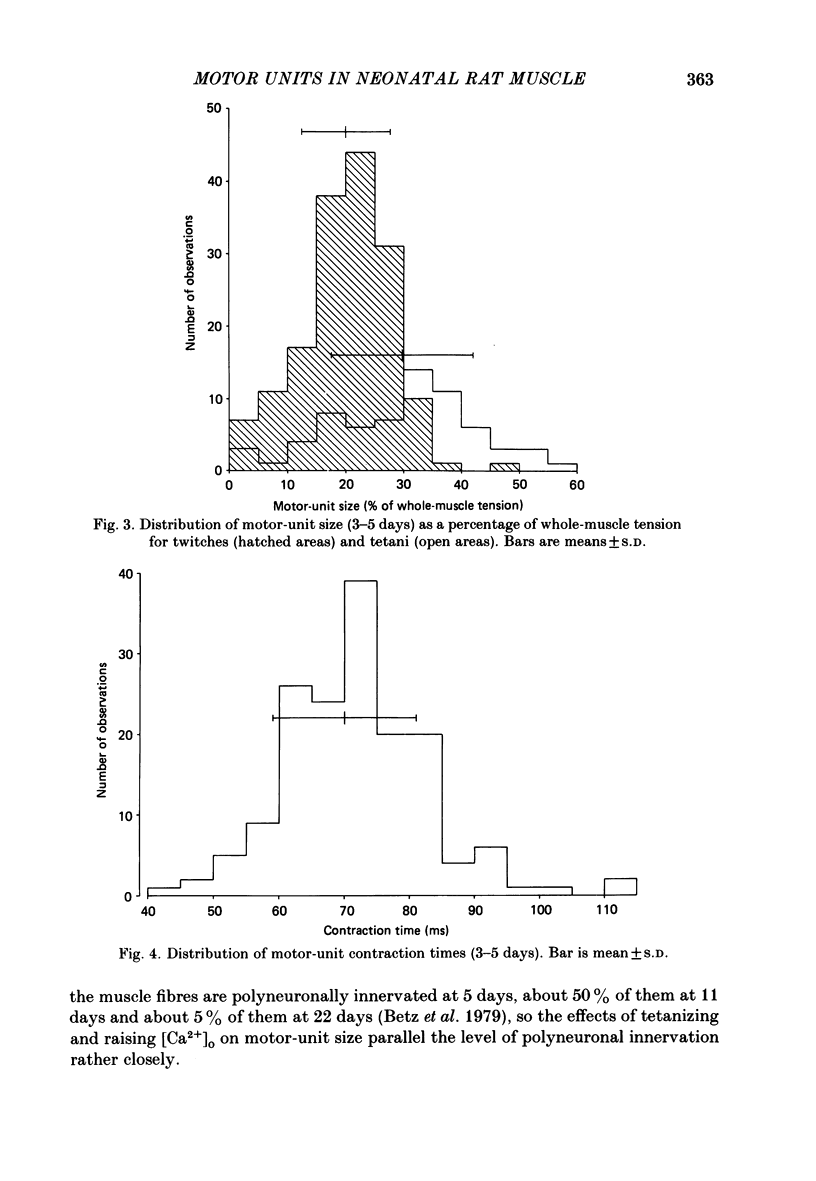






Selected References
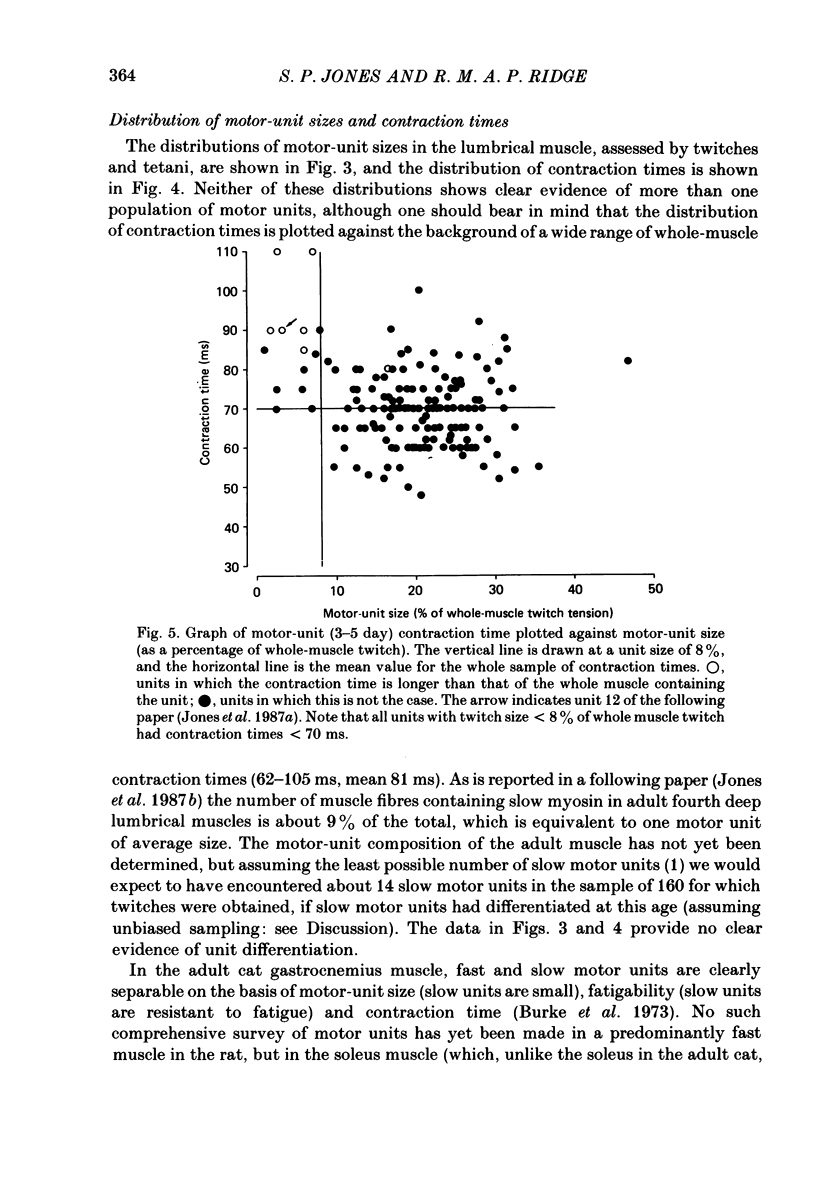
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D., Mallart A. Dual innervation of end-plate sites and its consequences for neuromuscular transmission in muscles of adult Xenopus laevis. J Physiol. 1979 Apr;289:203–218. doi: 10.1113/jphysiol.1979.sp012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal F., Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev. 1980 Jan;60(1):90–142. doi: 10.1152/physrev.1980.60.1.90. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistracher P., Hunt C. C. The relation of membrane changes ot contraction in twitch muscle fibres. J Physiol. 1969 May;201(3):589–611. doi: 10.1113/jphysiol.1969.sp008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. Rat muscle during post-natal development: evidence in favour of no interconversion between fast- and slow-twitch fibres. J Physiol. 1987 May;386:395–406. doi: 10.1113/jphysiol.1987.sp016540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. The non-selective innervation of muscle fibres and mixed composition of motor units in a muscle of neonatal rat. J Physiol. 1987 May;386:377–394. doi: 10.1113/jphysiol.1987.sp016539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons G. E., Haselgrove J., Kelly A. M., Rubinstein N. A. Myosin transitions in developing fast and slow muscles of the rat hindlimb. Differentiation. 1983;25(2):168–175. doi: 10.1111/j.1432-0436.1984.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Ranatunga K. W. Changes produced by chronic denervation in the temperature-dependent isometric contractile characteristics of rat fast and slow twitch skeletal muscles. J Physiol. 1977 Dec;273(1):255–262. doi: 10.1113/jphysiol.1977.sp012092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M., Betz W. J. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. J Neurosci. 1984 Oct;4(10):2614–2620. doi: 10.1523/JNEUROSCI.04-10-02614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Development of muscle fiber specialization in the rat hindlimb. J Cell Biol. 1981 Jul;90(1):128–144. doi: 10.1083/jcb.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H. Skeletal muscle fibers of newborn rats are coupled by gap junctions. Dev Biol. 1982 Jun;91(2):485–490. doi: 10.1016/0012-1606(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Sutton L. A., Riley D. A. Fibre type composition of single motor units during synapse elimination in neonatal rat soleus muscle. Nature. 1984 Jun 21;309(5970):709–711. doi: 10.1038/309709a0. [DOI] [PubMed] [Google Scholar]


