Abstract
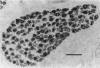
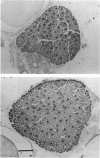
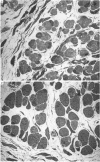
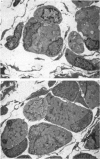
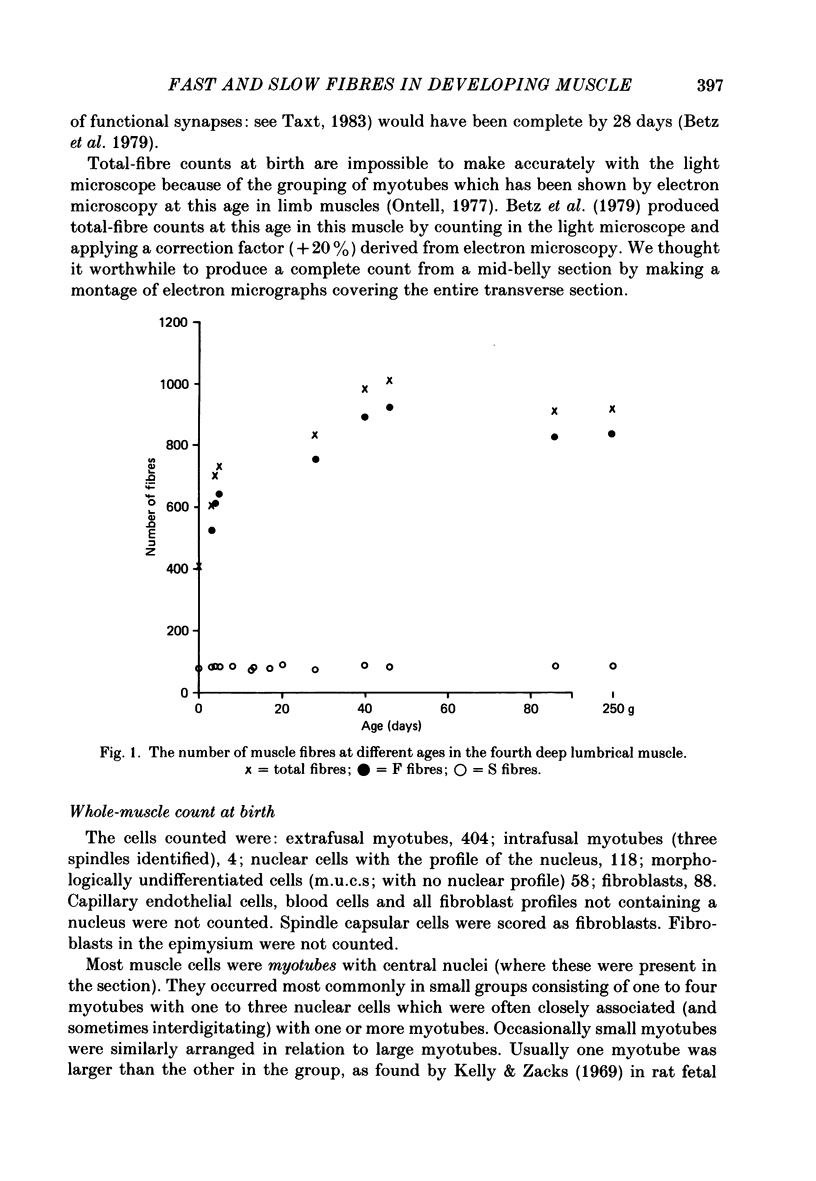
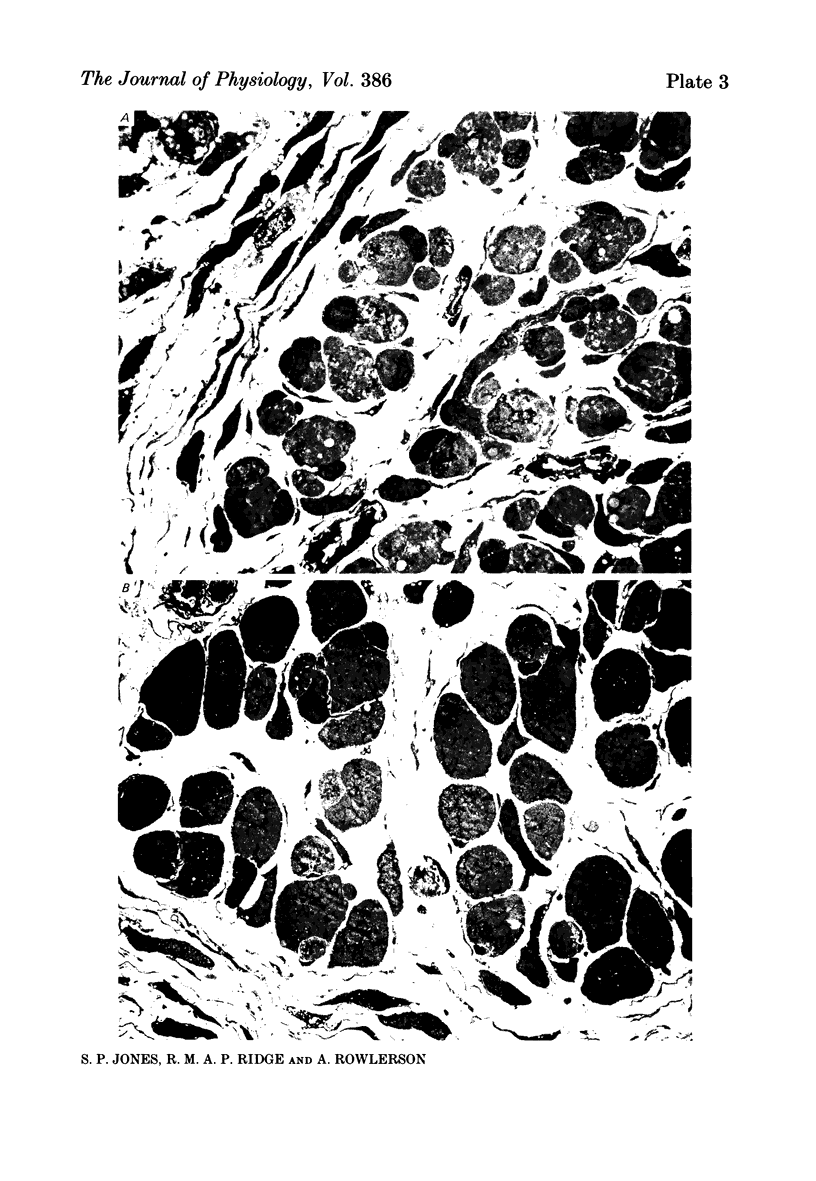
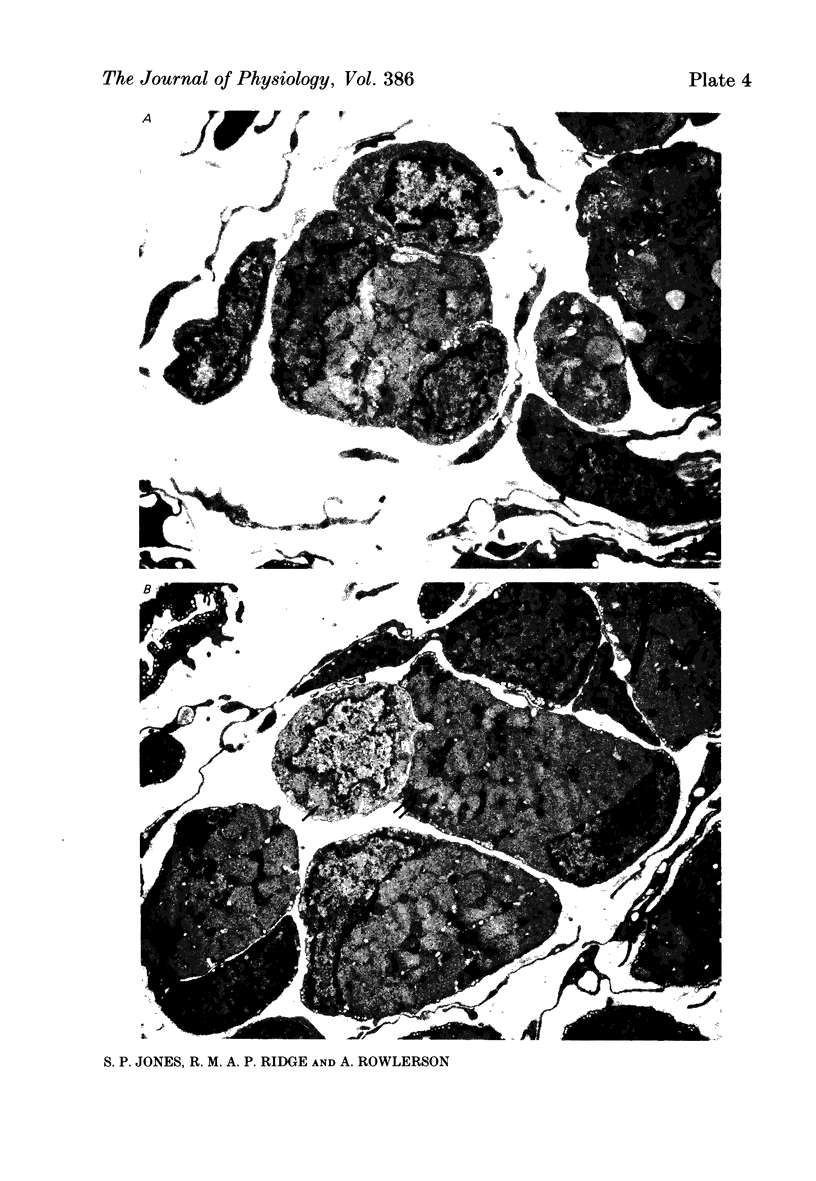
1. It was confirmed that in the fourth deep lumbrical muscle of the rat the number of muscle fibres at birth is about half that in the adult. 2. The average number of slow-myosin-containing (S) fibres (as determined by specific antibody binding) remains constant from birth to adulthood. Therefore it is likely that all the muscle fibres generated post-natally are type F (i.e. slow-myosin-free). 3. A comparison in the electron microscope between transverse mid-belly sections from new-born (day 0) and 4 day muscles showed many muscle fibres or myotubes to be intimately associated with other fibres and cells of other types in the new-born, but to be much less closely grouped at 4 days. 4. A full cell count was obtained from electron microscopy of a mid-belly section of a lumbrical muscle at birth. 5. Cross-sectional area measurements in the light microscope at 3-5 days and in the adult showed that at 3-5 days on average the S fibres have a greater cross-sectional area than the F fibres. This is reversed in the adult where the S fibres are the smaller. At 3-5 days the range of cross-sectional areas of F fibres is much wider than for S fibres. Some F fibres are among the largest fibres in the muscle. 6. It is argued from the data that the motor units of adult muscle, which are homogeneous with respect to muscle-fibre types, are produced by selective withdrawal of neonatal motor-unit contacts during developmental synapse elimination.
Full text
PDF








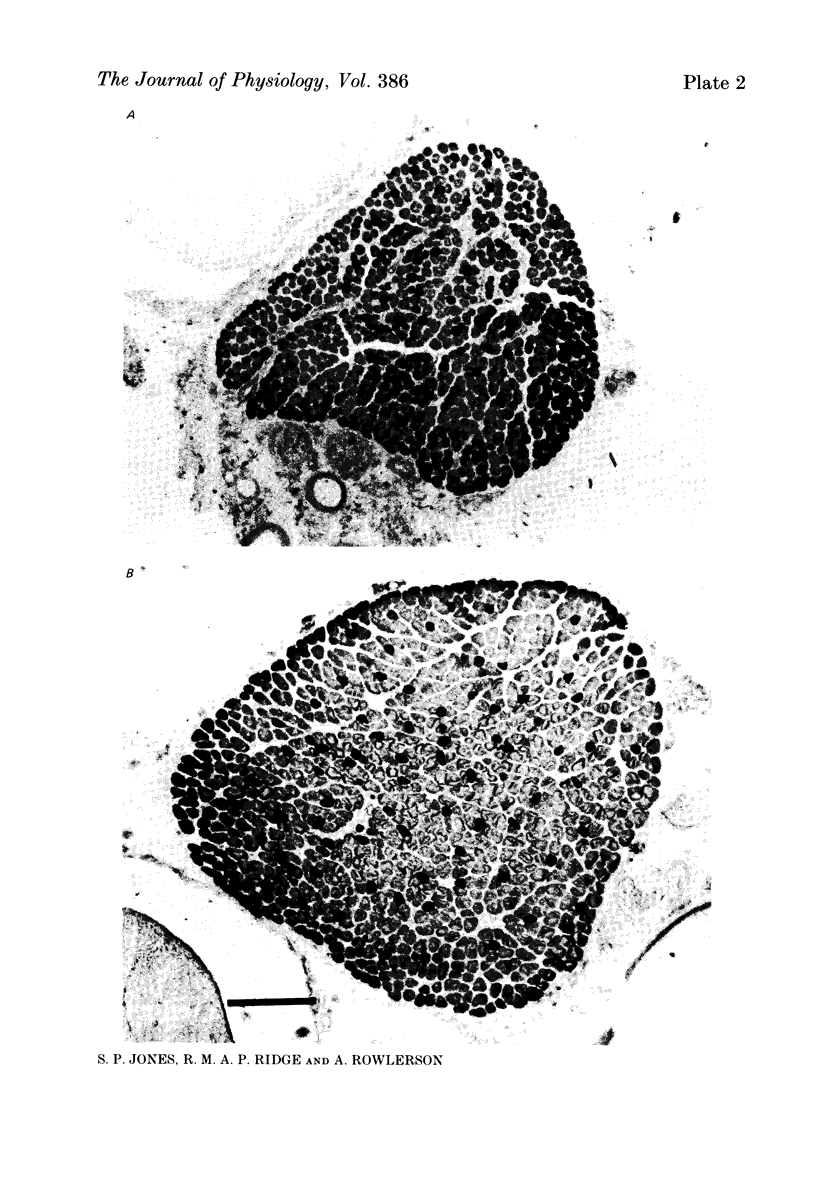
Images in this article
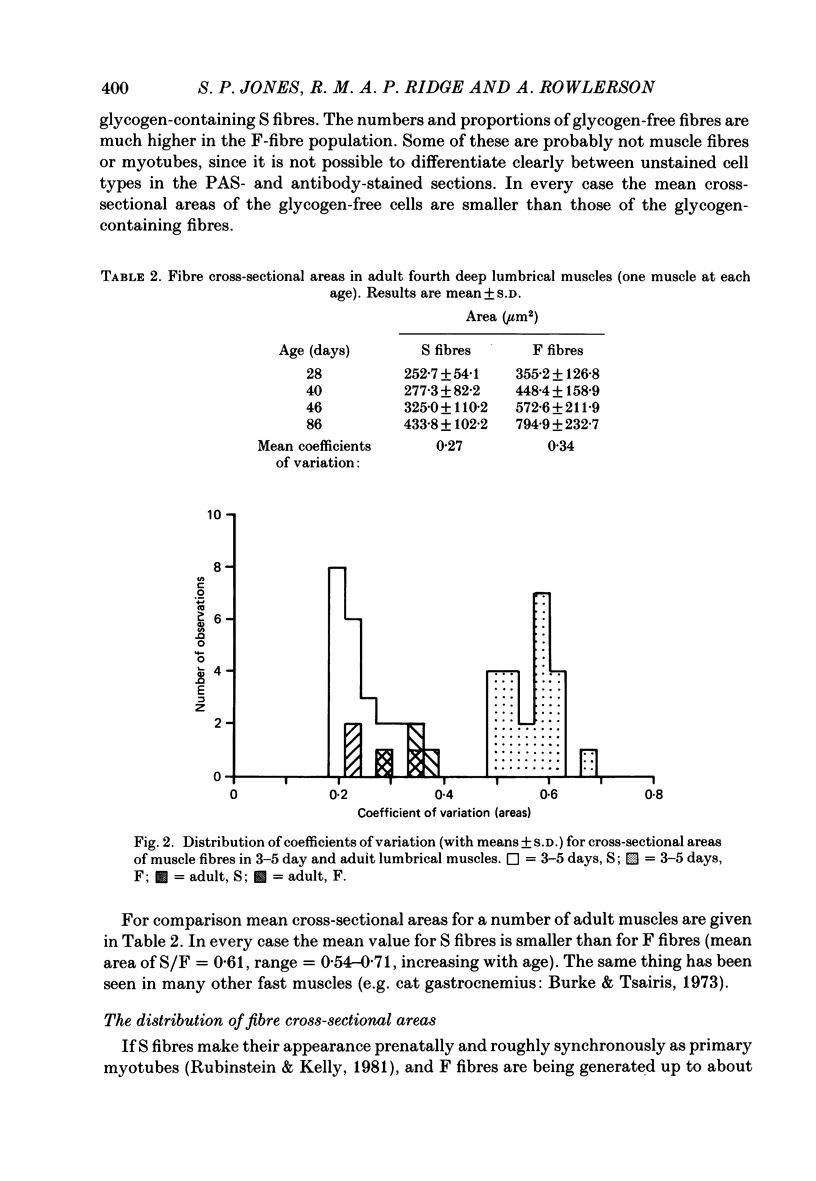
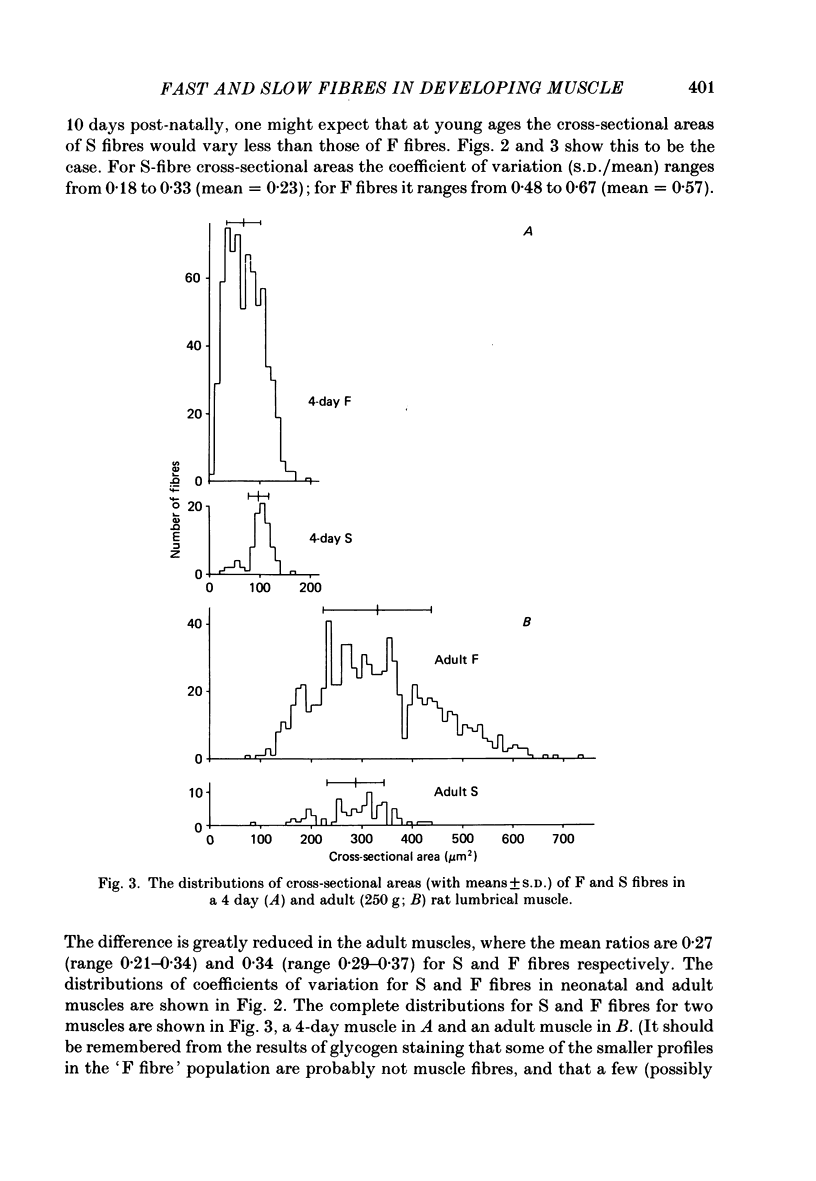
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. Segmental motor projections to rat muscles during the loss of polyneuronal innervation. Brain Res. 1984 Mar;315(1):1–7. doi: 10.1016/0165-3806(84)90070-1. [DOI] [PubMed] [Google Scholar]
- Bennett M., Lavidis N. Topographical projections of segmental nerves to the frog glutaeus muscle during loss of polyneuronal innervation. J Physiol. 1986 Jun;375:303–325. doi: 10.1113/jphysiol.1986.sp016118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Booth C. M. Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature. 1983 Aug 25;304(5928):741–742. doi: 10.1038/304741a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Yip J. W. Formation and elimination of foreign synapses on adult salamander muscle. J Physiol. 1978 Jan;274:299–310. doi: 10.1113/jphysiol.1978.sp012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde A., Huerta M., Stefani E. Selective reinnervation of twitch and tonic muscle fibres of the frog. J Physiol. 1983 Jul;340:513–524. doi: 10.1113/jphysiol.1983.sp014777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder N., Mills J., Tonge D. A. Selective reinnervation of skeletal muscle in the newt Triturus cristatus. J Physiol. 1982 May;326:371–384. doi: 10.1113/jphysiol.1982.sp014199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M. Motor units in a skeletal muscle of neonatal rat: mechanical properties and weak neuromuscular transmission. J Physiol. 1987 May;386:355–375. doi: 10.1113/jphysiol.1987.sp016538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. The non-selective innervation of muscle fibres and mixed composition of motor units in a muscle of neonatal rat. J Physiol. 1987 May;386:377–394. doi: 10.1113/jphysiol.1987.sp016539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The histogenesis of rat intercostal muscle. J Cell Biol. 1969 Jul;42(1):135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn A. Stages in the development of cat muscle spindles. J Embryol Exp Morphol. 1984 Aug;82:177–216. [PubMed] [Google Scholar]
- Ontell M., Dunn R. F. Neonatal muscle growth: a quantitative study. Am J Anat. 1978 Aug;152(4):539–555. doi: 10.1002/aja.1001520408. [DOI] [PubMed] [Google Scholar]
- Ontell M. Neonatal muscle: an electron microscopic study. Anat Rec. 1977 Dec;189(4):669–690. doi: 10.1002/ar.1091890410. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M., Betz W. J. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. J Neurosci. 1984 Oct;4(10):2614–2620. doi: 10.1523/JNEUROSCI.04-10-02614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Development of muscle fiber specialization in the rat hindlimb. J Cell Biol. 1981 Jul;90(1):128–144. doi: 10.1083/jcb.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H. Skeletal muscle fibers of newborn rats are coupled by gap junctions. Dev Biol. 1982 Jun;91(2):485–490. doi: 10.1016/0012-1606(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Taxt T. Local and systemic effects of tetrodotoxin on the formation and elimination of synapses in reinnervated adult rat muscle. J Physiol. 1983 Jul;340:175–194. doi: 10.1113/jphysiol.1983.sp014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Wigston D. J., Sanes J. R. Selective reinnervation of intercostal muscles transplanted from different segmental levels to a common site. J Neurosci. 1985 May;5(5):1208–1221. doi: 10.1523/JNEUROSCI.05-05-01208.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]