Abstract
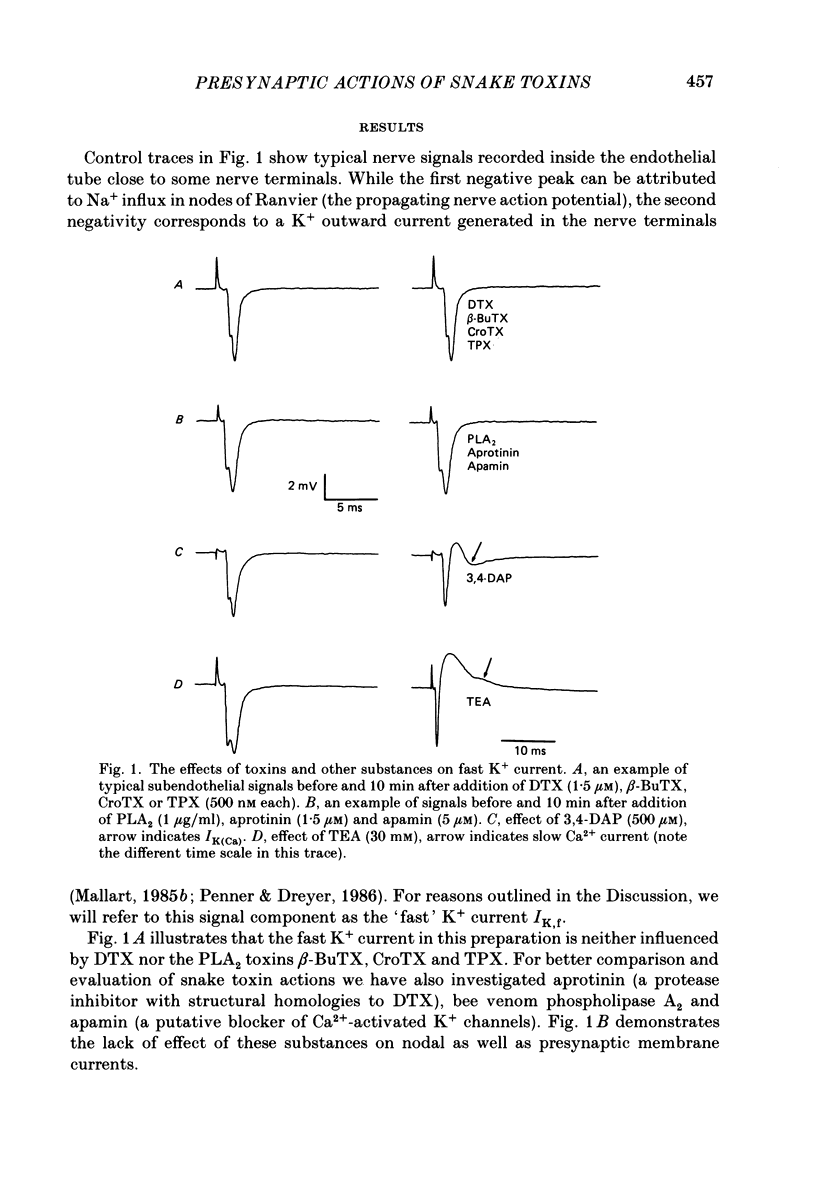
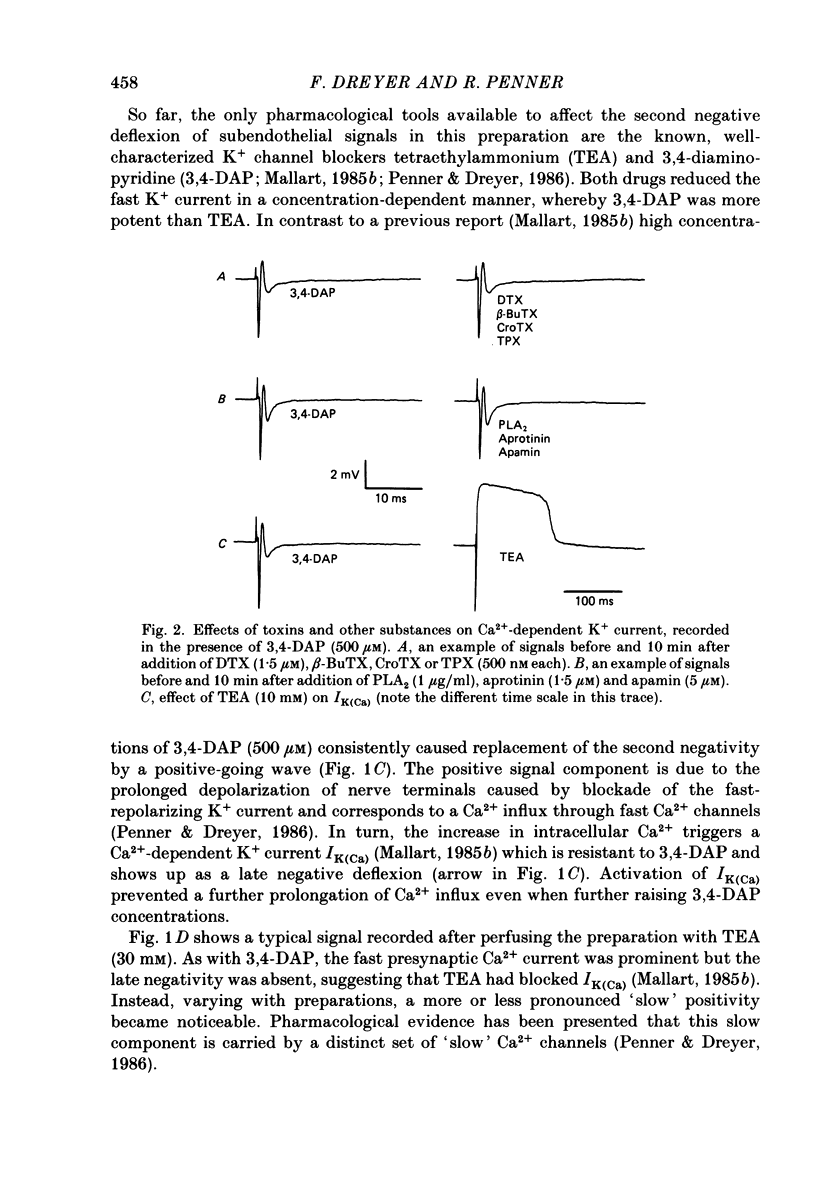
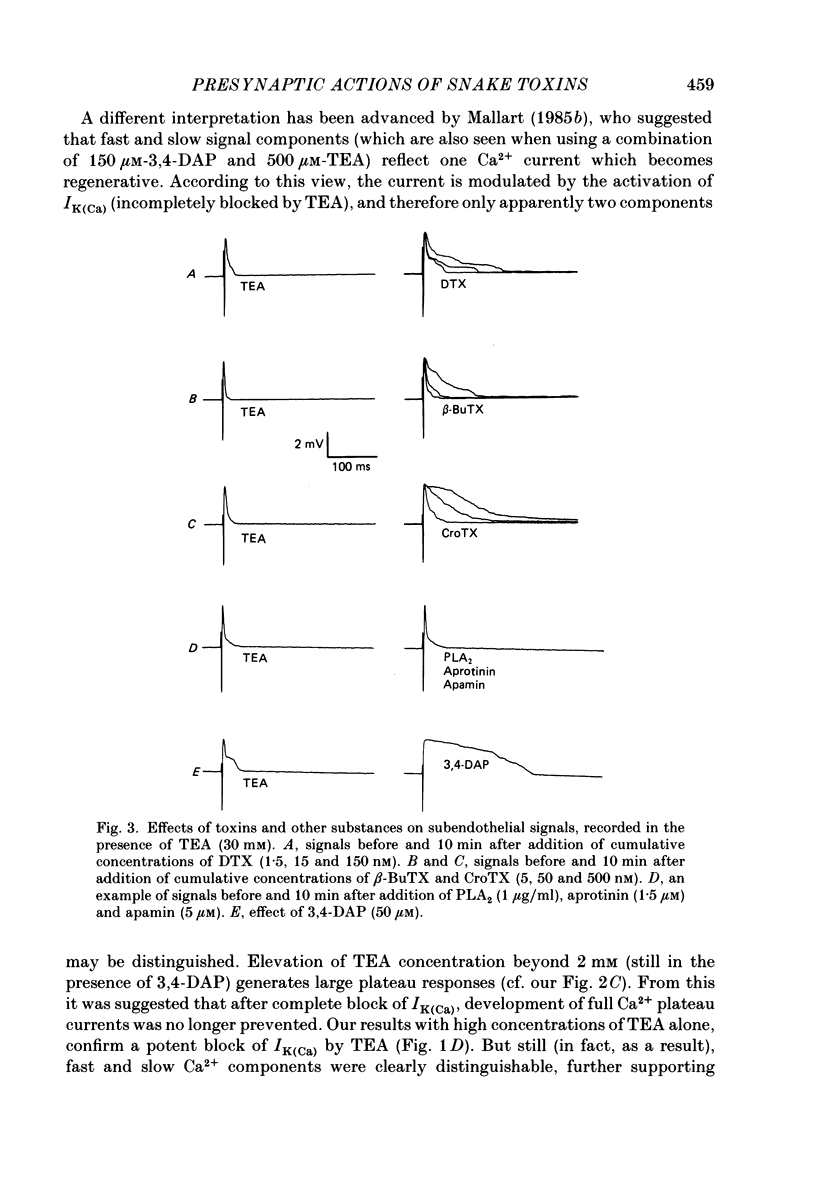
1. The m. triangularis sterni of the mouse was used to investigate the actions of dendrotoxin, beta-bungarotoxin, crotoxin, taipoxin, bee venom phospholipase A2, aprotinin and apamin on presynaptic currents which flow inside the perineural sheath of nerve bundles upon nerve stimulation. 2. Neither the fast K+ current (IK,f) nor the Ca2+-dependent K+ current IK(Ca) (unmasked after blockade of IK,f by 3,4-diaminopyridine) was affected by the neurotoxins and drugs mentioned. 3. Inhibition of both IK,f and IK(Ca) by tetraethylammonium (30 mM) prolonged presynaptic depolarization owing to Ca2+ influx through fast and slow Ca2+ channels. Additional application of dendrotoxin, beta-bungarotoxin, crotoxin or taipoxin in the nanomolar range caused further prolongation of Ca2+ influx, presumably due to blockade of slowly activating K+ current (IK,s). Onset of toxin effects was immediate and could not be reversed by washing for 60 min. 4. Similar prolongation of slow Ca2+ current was effected by 3,4-diaminopyridine, whereas addition of apamin, aprotinin or phospholipase A2 left the signals unchanged. 5. These data indicate that facilitatory actions of dendrotoxin, beta-bungarotoxin, taipoxin and crotoxin are mediated by an increase of Ca2+ entry into nerve terminals. The actions of these toxins are discussed in terms of a blockade of presynaptic K+ channels with slow activation kinetics.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Alemá S., Miledi R. Isolation and characterization of presynaptically acting neurotoxins from the venom of Bungarus snakes. Eur J Biochem. 1977 Oct 17;80(1):1–12. doi: 10.1111/j.1432-1033.1977.tb11849.x. [DOI] [PubMed] [Google Scholar]
- Abe T., Limbrick A. R., Miledi R. Acute muscle denervation induced by beta-bungarotoxin. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):545–553. doi: 10.1098/rspb.1976.0093. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Halliwell J. V., Black J. D., Williams R. S., Pelchen-Matthews A., Breeze A. L., Mehraban F., Othman I. B., Black A. R. Botulinum neurotoxin and dendrotoxin as probes for studies on transmitter release. J Physiol (Paris) 1984;79(4):280–303. [PubMed] [Google Scholar]
- Gundersen C. B., Katz B., Miledi R. The antagonism between botulinum toxin and calcium in motor nerve terminals. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):369–376. doi: 10.1098/rspb.1982.0080. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Othman I. B., Pelchen-Matthews A., Dolly J. O. Central action of dendrotoxin: selective reduction of a transient K conductance in hippocampus and binding to localized acceptors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):493–497. doi: 10.1073/pnas.83.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Karlsson E. Protease inhibitor homologues from mamba venoms: facilitation of acetylcholine release and interactions with prejunctional blocking toxins. Br J Pharmacol. 1982 Sep;77(1):153–161. doi: 10.1111/j.1476-5381.1982.tb09281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. D., Gundersen C. B., Jr Effects and mechanisms of polypeptide neurotoxins that act presynaptically. Annu Rev Pharmacol Toxicol. 1980;20:307–336. doi: 10.1146/annurev.pa.20.040180.001515. [DOI] [PubMed] [Google Scholar]
- Livengood D. R., Manalis R. S., Donlon M. A., Masukawa L. M., Tobias G. S., Shain W. Blockade of neuromuscular transmission by enzymatically active and inactive beta-bungarotoxin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1029–1033. doi: 10.1073/pnas.75.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. A calcium-activated potassium current in motor nerve terminals of the mouse. J Physiol. 1985 Nov;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. Electric current flow inside perineurial sheaths of mouse motor nerves. J Physiol. 1985 Nov;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Angaut-Petit D., Mallart A., Bournaud R., Faille L., Brigant J. L. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods. 1981 Aug;4(2):109–115. doi: 10.1016/0165-0270(81)90044-3. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Penner R., Petersen M., Pierau F. K., Dreyer F. Dendrotoxin: a selective blocker of a non-inactivating potassium current in guinea-pig dorsal root ganglion neurones. Pflugers Arch. 1986 Oct;407(4):365–369. doi: 10.1007/BF00652619. [DOI] [PubMed] [Google Scholar]
- Petersen M., Penner R., Pierau F. K., Dreyer F. Beta-bungarotoxin inhibits a non-inactivating potassium current in guinea pig dorsal root ganglion neurones. Neurosci Lett. 1986 Jul 11;68(1):141–145. doi: 10.1016/0304-3940(86)90244-2. [DOI] [PubMed] [Google Scholar]
- Rehm H., Betz H. Solubilization and characterization of the beta-bungarotoxin-binding protein of chick brain membranes. J Biol Chem. 1984 Jun 10;259(11):6865–6869. [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Halliwell J. V., Brown D. A. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurones by blocking a slowly inactivating outward current. Neurosci Lett. 1986 Mar 14;64(3):299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- Weller U., Bernhardt U., Siemen D., Dreyer F., Vogel W., Habermann E. Electrophysiological and neurobiochemical evidence for the blockade of a potassium channel by dendrotoxin. Naunyn Schmiedebergs Arch Pharmacol. 1985 Aug;330(2):77–83. doi: 10.1007/BF00499898. [DOI] [PubMed] [Google Scholar]


