Abstract
Background
Phenotypic traits are indicative of a plant’s resource utilization and survival strategies. Understanding the geographical differentiation of Calamus rhabdocladus phenotypes holds significant theoretical and practical value for genetic breeding and the selection of superior geographical provenances. This study analyzed the geographic variation in 15 phenotypic traits of leaves, stems, roots, flagellums, and other parts from 10 provenances of C. rhabdocladus in China. The phenotypic characters of C. rhabdocladus were measured in the field, while soil factors were collected in the field and analyzed in the laboratory. Climatic data were obtained through online sources. Pearson’s correlation was used to analyze the relationships between the 15 phenotypic traits. Redundancy analysis and variance decomposition analysis were used to quantify the effects of climatic and soil factors on the geographical variation in phenotypic traits of C. rhabdocladus, and the key influencing factors were obtained by comparing the explanatory rates of soil and climatic factors on phenotypic traits of C. rhabdocladus.
Results
The results showed that the Coefficient of variation (CV) of phenotypic traits among provenances ranges from 7.48 to 182.16%, with root dry weight exhibiting the largest variation. The plasticity indices varied between 0.16 and 0.82, with the flagellum volume showing the highest value. Significant correlations were observed among leaf, stem, flagellum, and root traits. Average air temperature was identified as the crucial climatic factor influencing the phenotypic traits of C. rhabdocladus, while effective nitrogen and organic matter content were the key soil factors influencing these traits. Within the scope of this study, climatic factors provided a better explanation of phenotype variation than soil factors.
Conclusions
These results highlight the importance of climatic adaptation in shaping phenotypic diversity and suggest that future research should explore the genetic mechanisms underlying these traits and their interactions with environmental factors at finer spatial scales.
Keywords: Geographical variation, Phenotypic traits, Climate factors, Soil factors, Trait plasticity, Calamus rhabdocladus
Introduction
Plant traits encompass morphological, anatomical, biochemical, physiological, or phenological features that are measured at the individual level [1, 2]. Plant functional traits are key plant properties closely linked to plant colonization, growth, survival, and mortality during long-term evolutionary adaptation [3, 4]. These functional traits are typically interrelated and co-evolved, reflecting how plants respond and adapt to external environmental conditions, and can contribute to determining a plant’s ecosystem functionality [5, 6]. At local scales, functional traits are often used to assess plant growth status, and at larger spatial scales, species exhibit changes in functional traits along gradients of abiotic or biotic factors, driven by natural selection or phenotypic plasticity [7–9]. As a crucial link between plants, the environment, and ecosystems, functional traits are also indicative of a plant’s survival strategies [10–11]. Leaves, as the primary organ for photosynthesis and plant material production [4], play a critical role in resource acquisition and utilization [3]. Leaf functional traits are highly sensitive to environmental changes and are relatively easier to measure compared to other plant traits. Notably, the Specific leaf area (SLA) and the leaf dry matter content (LDMC) are considered key predictors of plant strategies [12, 13]. The concept of the leaf economic spectrum (LES), introduced through a global analysis of 2548 plant leaf traits, quantifies the trade-off strategies of plants on a global scale [14]. While LES has been widely confirmed across various spatial, temporal, and ecosystem contexts [5], significant gaps remain in understanding how these traits vary and interact in specific environments, such as extreme or heterogeneous habitats. For instance, Yang et al [15]. Demonstrated high variability in leaf size-related traits among 332 species on the Qinghai-Tibet Plateau, with leaf area showing the greatest variation. Similarly, Wang et al [16]. Found extensive spatial heterogeneity and strong correlations among leaf functional traits along altitudinal gradients in Abies acanthus. However, the mechanisms driving these patterns and their implications for plant adaptation remain poorly understood.
Extending beyond leaves, similar economic spectra have been proposed for stems and roots. In the wood economic spectrum (WES), wood density (WD) represents a key functional trait mediating trade-offs between growth and survival [12, 17]. In the root economic spectrum, traits including root diameter, root length, and root tissue density reflect nutrient and water acquisition strategies [18, 19]. Despite these advances, integrated studies linking leaf, stem, and root traits across diverse environments are scarce, limiting our understanding of whole-plant adaptation strategies.
Recently, the relationship between plant functional traits and the environment has emerged as a key topic in functional biogeography and macroecological studies. At the global scale, climate impacts on plant functional traits are particularly significant, with annual mean temperature and precipitation identified as its major drivers. In warm and humid low latitudes, leaves tend to be larger, whereas in arid and cold high latitudes, plants typically have smaller leaves [20]. At the local scale, soil nutrients exert a more pronounced influence on plant functional traits, driving changes in leaf and wood traits, with species growing faster in nutrient-rich habitats [21]. However, the relationship between functional traits and environmental factors is not fixed and may vary across different regions or species. For example, leaf area and quality decrease with warming, while the leaf aspect ratio increases [22]. In cold and low rainfall temperate climate zones, deciduous trees usually exhibit high SLA and low WD, whereas in tropical zones with high temperature and high rainfall, evergreen trees tend to have low SLA and higher LDMC, enhancing their resource utilization efficiency, resistance to disease and insect pest erosion, and resource acquisition [21].
Yang et al [23]. Found that in the arid areas of northwest China, under the stress of arid environment, rainfall is the main climate driving factor affecting the development of plant leaves. Zhao et al [24]. Through a decade-long monitoring study in Hainan’s tropical cloud forests, found that plant functional traits exhibited a stronger response to soil nutrients over time, highlighting the high sensitivity of these traits to environmental changes at the individual level. In karst environments, wood density among tree species varies with habitat; karst hilltop species with less soil substrate have higher WD than valley species, which benefit from higher soil moisture content [25]. Despite these findings, critical gaps remain in understanding how these patterns generalize across different ecosystems and species. Additionally, the role of genetic adaptation versus phenotypic plasticity in driving these responses remains unclear. It is necessary to investigate in the future how functional traits respond to combined stressors such as drought and nutrient limitation, as well as how these responses influence plant survival and ecosystem resilience under climate change.
Calamus rhabdocladus Burret, a species in the genus Calamus of the Palmae family, is a common climbing companion plant in tropical and subtropical forests. It has a wide geographic distribution, found in the Chinese provinces of Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan, as well as in Vietnam and Laos. C. rhabdocladus canes are used in furniture making, while its shoots and fruits are edible, contributing to the species’ economic importance [26]. Plants undergo morphological differentiation in response to different geographic conditions and develop distinct adaptations based on the climate and soil [13, 27]. Despite its important economic significance, the germplasm collection and morphological difference assessment of C. rhabdocladus are partial and incomplete. The specific effects of climate and soil on the phenotypic traits of C. rhabdocladus remains unclear. In this study, we investigated the geographic variation of phenotypic traits and the effects of climate and soil factors on the phenotypic traits of C. rhabdocladus, aiming to understand (1) the geographic variation and correlations among phenotypic traits in the C. rhabdocladus (2) the key climatic and soil factors affecting these traits, and (3) whether climatic or soil factor primarily influence the phenotypic traits of C. rhabdocladus. The results of this study has important theoretical and practical implications for genetic breeding and the selection of superior geographic seed sources of C. rhabdocladus.
Materials and methods
Division of study area and plots
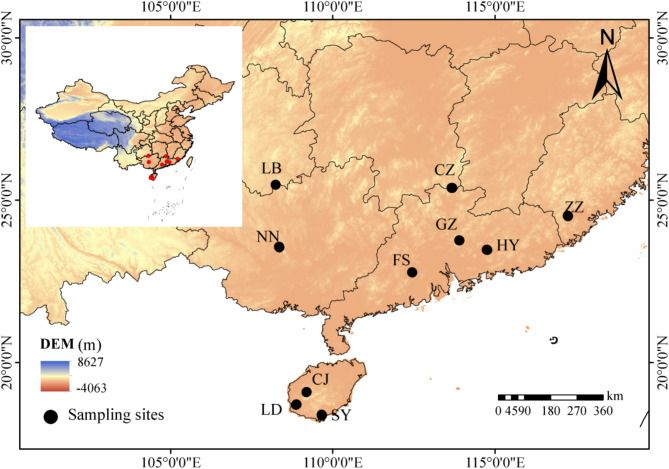
Sampling was conducted during July–August 2022 and July–August 2023 among 10 provenance sites spanning six provinces and regions in China: Hainan, Guangdong, Guangxi, Fujian, Guizhou, and Hunan. These sites cover the main distribution areas of C. rhabdocladus in China and are primarily located in tropical and subtropical regions characterized by distinct dry and wet seasons, thus being selected for this study. The sampling period was chosen to coincide with the mature growth phase of C. rhabdocladus, during which individual traits are relatively stable. Due to its wide distribution, 6 provenances were investigated in the first year and 4 additional samples were collected in the second year. The locations and basic information of the sampling sites are shown in a map and the following table (Fig. 1; Table 1). At each provenance site, 5 plants in good growth conditions and with cane lengths greater than 5 m were selected. This threshold ensures that the plants have reached maturity and exhibit stable traits. The selection of five individuals per site aligns with the general recommendation for the minimum sample size required to quantify species-specific trait values [28]. To ensure spatial independence, the straight-line distance between selected plants was maintained at more than 100 m. A circular sample plot with a radius of 8 m was established, centered on the selected plants. Within these plots, the growth of tree species (diameter at breast height > 10 cm) was recorded and a diameter tape was used to measure the DBH of the trees. Geographical information including longitude, latitude, elevation, and other geographical information of the plot center was recorded using a Garmin 639csx handheld GPS locator. Additionally, habitat characteristics including stand depression, slope, slope position, and slope direction were documented.
Fig. 1.
Distribution of sampling sites
Table 1.
Basic overview of sampling sites and their key characteristics
| Provenance | Abbreviation | Mean temperature of air (℃) | Average precipitation (mm) | Air temperature in January (℃) | Air temperature in July air (℃) |
|---|---|---|---|---|---|
| Sanya, Hainan | SY | 23.1 | 1635 | 20 | 28 |
| Ledong, Hainan | LD | 24.8 | 1566 | 19.4 | 27.3 |
| Changjiang, Hainan | CJ | 25.6 | 1742 | 13.5 | 22.8 |
| Heyuan, Guangdong | HY | 21.7 | 1619 | 13.3 | 28 |
| Guangzhou, Guangdong | GZ | 21.7 | 2077 | 13.9 | 28.8 |
| Foshan, Guangdong | FS | 23.1 | 1951 | 13.4 | 28.5 |
| Nanning, Guangxi | NN | 21.8 | 1723 | 5.8 | 21.9 |
| Zhangzhou, Fujian | ZJ | 22.1 | 1622 | 8.7 | 24.4 |
| Libo, Guizhou | LB | 19.3 | 1299 | 5.9 | 27.0 |
| Chenzhou, Hunan | CZ | 17.5 | 1626 | 6.5 | 29.7 |
Sample collection and trait measurement
Plant samples of C. rhabdocladus were collected in the field. Each plant was manually separated from its supporting wood and laid flat on a flat surface. Three mature, healthy, disease- and pest-free leaves were randomly selected from the plant. The chlorophyll SPAD values of 10 randomly chosen leaflets were measured using a chlorophyll meter (SPAD-502 Plus, Konica Minolta, Japan) while the leaves were still fresh. The average SPAD value of the 10 leaflets was calculated and used as the chlorophyll SPAD value for each leaf. After chlorophyll measurement, the leaves were placed in a bag and transported to the laboratory for the measurement of leaf functional traits. The canes were truncated from the base of the stem, and the number of leaves and nodes on each cane were counted. The length between the nodes was measured with a tape measure. Stem segments, each 5 cm in length, were cut from the cane at positions 1/4, 1/2 and 3/4 of the total length, placed in self-sealing bags and brought to the laboratory for the measurement of stem phenotypic traits. Three flagella used for climbing were randomly selected, stripped from the cane plants, loaded in self-sealing bags, and brought back to the laboratory for the measurement of flagella functional traits. Soil samples containing root systems were collected using a 70 mm diameter, 20 cm deep root drill, positioned 15 cm from the plant. The soil was divided into three layers: 0–20 cm, 20–40 cm, and 40–60 cm. Roots were extracted from each layer of soil, washed with water, and brought back to the laboratory in self-sealing bags for measurement of root phenotypic traits (Table 2).
Table 2.
Description of phenotypic traits, soil, and Climatic factors
| Phenotypic trait | Abbreviation | Units | Soil and climate factors | Abbreviation | Units | |
|---|---|---|---|---|---|---|
| Leaf dry weight | LDW | g | Potential of hydrogen | pH | / | |
| Specific leaf area | SLA | cm2 g− 1 | Organic matter content | OM | g kg− 1 | |
| Leaf dry matter content | LDMC | g g− 1 | Total nitrogen content | N | g kg− 1 | |
| Leaf sheath dry matter content | LSDMC | g g− 1 | Total phosphorus content | P | g kg− 1 | |
| Pinnated leaf number | PN | pieces | Alkaline nitrogen content | AN | mg kg− 1 | |
| Soil and plant analyzer development | SPAD | / | Quick-acting phosphorus content | AP | mg kg− 1 | |
| Node number | NN | number | Average temperatures | Ave tem | ℃ | |
| Internode length | II | cm | Effective cumulative temperature | effect.tem | ℃ | |
| Stem dry weight | SDW | g | Maximum temperature | max.tem | ℃ | |
| Wood density | WD | g cm− 3 | Minimum temperature | min.tem | ℃ | |
| Flagellum volume | FV | cm3 | Relative humidity | rela.humid | % | |
| Flagellum dry matter content | FDMC | g g− 1 | Measured quantity of rain | precipitation | mm | |
| Root dry weight | RDW | g | Solar radiation intensity | solar.radia | w m− 2 | |
| Root dry matter content | RDMC | g g− 1 | ||||
| Root density | RD | g cm− 3 |
The leaves, stems, sheaths, whips, and roots collected in the wild were measured within 24 h of being brought back to the laboratory. Leaf area was determined using the photographic method: a digital camera was used to vertically capture an image of the entire leaf alongside a reference object. The number of pixels in the leaf and reference images were measured using Photoshop CS5 software, and the leaf area was calculated by comparing the pixel count to the actual area of the reference.
The main phenotypic traits were calculated as follows:
Specific leaf area (SLA) = leaf area/dry leaf mass..
Leaf dry matter content (LDMC) = dry leaf mass/fresh leaf mass..
Leaf sheath dry matter content (LSDMC) = dry leaf sheath mass/fresh leaf sheath mass..
Wood density (WD) = dry stem segment mass/stem segment volume..
Flagellum dry matter content (FDMC) = dried flagellum mass/fresh flagellum mass..
Root dry matter content (RDMC) = dry root mass/fresh root mass;.
Root density (RD) = dry root mass/root volume..
Measurement of soil samples
Soil samples from three layers at each sampling site, collected using a root auger, were placed in a ventilated area in the laboratory to dry naturally. Debris including roots, stones, and plant and animal residues were removed. The dried soil was the milled, pulverized, and passed through a 0.149 mm soil sieve. Six soil factors were determined: pH, organic matter (OM) content, total nitrogen content, total phosphorus content, alkali-dissolved nitrogen content, and quick-acting phosphorus content (Table 2). Soil pH was measured using the glass electrode method. Organic matter content was determined by the potassium dichromate oxidation-external heating method, while the total nitrogen content was measured using the Kjeldahl method. The total phosphorus and effective phosphorus contents were determined by the molybdenum-antimony anticolorimetric method. Lastly, alkali-dissolved nitrogen content was measured using the alkaline diffusion method.
Acquisition of climate data
Climate data for the provenance sites were obtained through the National Meteorological Administration of China (http://data.cma.cn/). Seven climatic factors were collected for each site, including mean temperature (℃), effective accumulated temperature (℃), maximum temperature (℃), minimum temperature (℃), relative humidity (%), precipitation (mm), and solar radiation intensity (Wm− 2). The data covered a 10-year period from 2012 to 2021.
Statistical analysis
Data was recorded and preprocessed using Excel 365. Descriptive statistical analysis of the phenotypic trait data was performed using the ‘describe()’ function in R 4.2.2, specifically within the “pych” package. The coefficient of variation (CV) for each trait was calculated using the formula CV= (SE / Mean) × 100%. The plasticity index (PI) was calculated using the formula PI = (maximum value − minimum value) / maximum value [29]. The correlation between cane phenotypic traits was analyzed using Pearson’s method. Redundancy analysis (RDA) was performed using the ‘rda()’ function in the “vegan” package to examine the relationship between climatic conditions, soil factors, and leaf functional traits of the provenances. Permutation tests for the significance for the RDA model and for each axis were conducted using the ‘anova()’ function. Forward selection was carried out using the using the ‘ordistep()’ function, with 999 random permutations to test the marginal role of the explanatory variables. The importance of R2 in explaining phenotypic trait variation according to the explanatory variables was ranked. The ‘vif.’ function was used to perform a collinearity test within the ‘cca()’ function. Variance partitioning was conducted using the ‘varpart()’ function, and the ‘ggplot2’ package was used for mapping.
Results
Phenotypic trait variation in C. rhabdocladus provenances
Fifteen phenotypic traits were statistically analyzed for 10 provenances of C. rhabdocladus (Table 3). The coefficient of variation among provenances ranged from 7.48 to 182.16%, with the largest variation observed in root dry weight, followed by flagellum volume, and the smallest variation in flagellum matter content. The threshold for the coefficient of variation is generally considered to be 20% [30–31]. The coefficients of variation of leaf dry weight, feather number, cane number, internode length (IL), stem weight, flagellum volume, root dry weight (RDW), root dry matter content, (RDMC) and root density (RD) were all above 20%, indicating a rich diversity of phenotypes. In contrast, the coefficients of variation for leaf matter content, specific leaf area, leaf sheath matter content, chlorophyll SPAD value, wood density, and flagellum matter content were below 20%, suggesting that these six traits exhibit are less diverse among provenances.
Table 3.
Descriptive statistical analysis of phenotypic traits among provenances
| SN | Traits | Min | Max | Mean | SD | CV | plasticity index |
|---|---|---|---|---|---|---|---|
| 1 | LDW | 7.02 | 131.18 | 43.73 | 26.45 | 60.47% | 0.68 |
| 2 | LDMC | 0.42 | 0.64 | 0.53 | 0.04 | 7.68% | 0.16 |
| 3 | SLA | 78.58 | 186.17 | 122.68 | 22.71 | 18.51% | 0.40 |
| 4 | LSDMC | 0.29 | 0.65 | 0.51 | 0.06 | 12.64% | 0.24 |
| 5 | PN | 4 | 42 | 21 | 10.35 | 49.02% | 0.63 |
| 6 | SPAD | 44.76 | 70.12 | 55.64 | 6.1 | 10.88% | 0.18 |
| 7 | NN | 10 | 202 | 60 | 35.72 | 59.29% | 0.64 |
| 8 | IL | 7.07 | 24.18 | 12.81 | 3.45 | 26.94% | 0.36 |
| 9 | SDW | 2.39 | 42.82 | 11.15 | 6.69 | 59.99% | 0.70 |
| 10 | WD | 0.31 | 0.83 | 0.56 | 0.09 | 15.66% | 0.29 |
| 11 | FV | 3.00 | 374 | 82 | 71.46 | 87.23% | 0.82 |
| 12 | FDMC | 0.44 | 0.62 | 0.52 | 0.04 | 7.48% | 0.17 |
| 13 | RDW | 0.03 | 51.21 | 6.05 | 11.03 | 182.16% | 0.69 |
| 14 | RDMC | 0.22 | 0.7 | 0.36 | 0.1 | 28.9% | 0.22 |
| 15 | RD | 0.14 | 0.6 | 0.32 | 0.1 | 30.29% | 0.32 |
The plasticity index threshold is generally considered to be 0.20 [32]. The plasticity indices of flagellum volume (FV), stem dry weight, root dry weight, leaf dry weight, node number (NN), and number of pinnate leaves (PN) were all above 0.60, indicating that these phenotypic traits are highly plastic and environmentally adaptable. In contrast, the plasticity indices of leaf dry matter content, flagellum dry matter content, and chlorophyll SPAD value were all lower than 0.20, suggesting that these phenotypic traits were less diverse and less influenced by environmental conditions.
Phenotypic trait correlation in C. rhabdocladus provenances
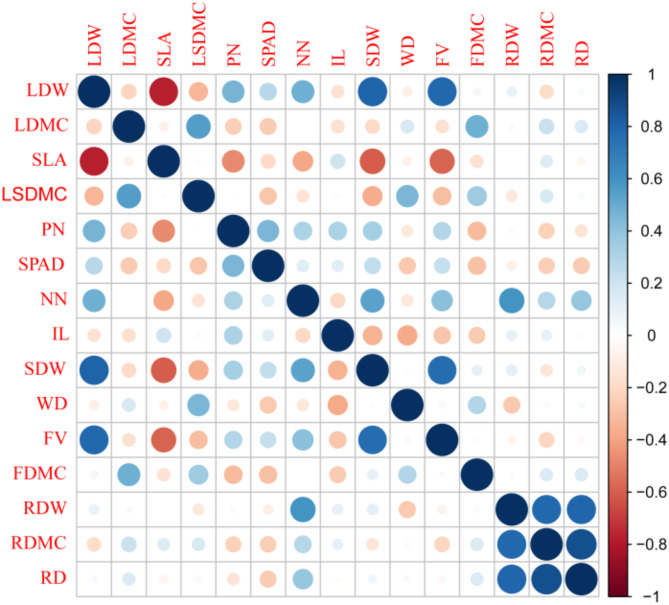
Pearson’s correlation analysis of the phenotypic traits of C. rhabdocladus showed varying degrees of significant correlations among leaf, stem, flagellum, and root traits (Fig. 2). LDW showed a very significant negative correlation with SLA and LSDMC (p < 0.001) but a very significant positive correlation with PN, SPAD, NN, SDW (p < 0.001), and FV. LDMC has a very significantly positive correlation with both LSDMC and FDMC (p < 0.001). SLA was significantly negatively correlated with PN, NN, SDW, and FV (p < 0.01). LSDMC had a very significant negative correlation with both SDW and FV (p < 0.001) but showed a very significantly positive correlation with WD and FDMC (p < 0.001). PN exhibited an extremely significant positive correlation with SPAD, NN, IL, SDW, and FV (p < 0.001), and very significant negative correlation with FDMC (p < 0.001). SPAD had a very significant negative correlation with FDMC (p < 0.001). NN showed an extremely significant positive correlation with SDW, FV, RDW, RDMC, and RD (p < 0.01). IL showed a very significant negative correlation with SDW and WD (p < 0.001). SDW and FV were significantly positively correlated with each other (p < 0.01). WD was positively correlated with TDMC (p < 0.05). RDW showed a very significant positive correlation with RDMC and RD (p < 0.001), and RDMC was also very significantly positively correlated with RD (p < 0.001).
Fig. 2.
Heat map of correlation among 15 phenotypic traits of C. rhabdocladus.The phenotypic traits represented by the English letters in the figure are shown in Table 2. The size of the dots and the intensity of the color represent the correlation between the two traits. Larger dots and darker colors indicate stronger correlations, with blue representing positive correlations and red representing negative correlations
Critical climatic drivers of phenotypic traits in C. rhabdocladus
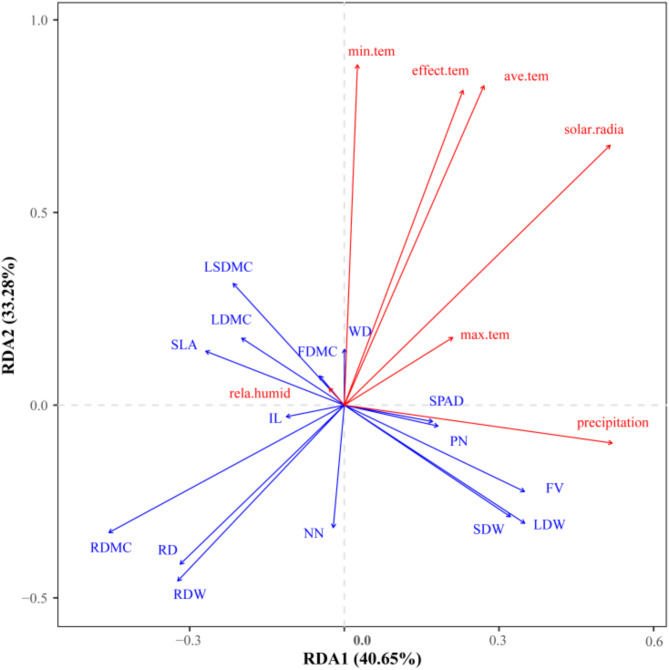
The results of RDA for climate factors and phenotypic traits show that the eigenvalue on the first axis is 40.65%, on the second axis is 33.28%, and together the first two axes explain 73.93% of the total variance (Fig. 3). Normally, a redundancy analysis interpretation rate of 70% or higher indicates a more reliable result. This suggests that the influence of climate on phenotypic traits is well captured by the RDA model. According to the ranking diagram, several key relationships were identified: the lowest temperature is closely associated with wood density and the number of rattan nodes; mean temperature, effective temperature, accumulated temperature, solar radiation intensity, and maximum temperature are strongly linked to root traits; and rainfall is closely related to feather number and chlorophyll SPAD value.
Fig. 3.
Biplot of redundancy analysis between climatic factors and phenotypic traits of C. rhabdocladus
The corrected R2 of the RDA model for climate and phenotypic traits was 0.434, which meant that 43.4% of the total variance in phenotypes of C. rhabdocladus can be explained by climate factors. The results of the covariance test showed a strong covariance between average temperature and effective cumulative temperature, both of which had a similar degree of influence on the phenotypes of C. rhabdocladus. However, the remaining climate factors exhibited weak covariance (Table 4). The effects of average temperature, effective cumulative temperature, minimum temperature, relative humidity, precipitation, and solar radiation intensity on the phenotypic traits of C. rhabdocladus were highly significant (P < 0.001), while the effect of maximum temperature on was significant (0.001 < P < 0.01). The R2 (explanation rate) assigned to each explanatory variable was obtained through RDA. The climatic factors were ranked in order of importance according to R2 size as follows: average temperature > effective cumulative temperature > maximum air temperature > precipitation > minimum air temperature > relative humidity > solar radiation intensity. Based on these findings, average temperature was identified as the key climatic factor influencing the phenotypic traits of C. rhabdocladus.
Table 4.
Redundancy analysis of climate factors and phenotypic traits
| Climatic factor | VIF | F | P | R 2 | sort |
|---|---|---|---|---|---|
| Ave tem | 1091.333 | 30.249 | 0.001*** | 0.116 | 1 |
| effect. tem | 751.354 | 23.440 | 0.001*** | 0.090 | 2 |
| max.tem | 15.376 | 4.251 | 0.002** | 0.075 | 3 |
| min. tem | 32.584 | 19.930 | 0.001*** | 0.044 | 5 |
| rela. humid | 6.075 | 12.466 | 0.001*** | 0.026 | 6 |
| precipitation | 1.674 | 9.266 | 0.001*** | 0.065 | 4 |
| solar. radia | 13.774 | 14.944 | 0.001*** | 0.018 | 7 |
Critical soil drivers of phenotypic traits in C. rhabdocladus
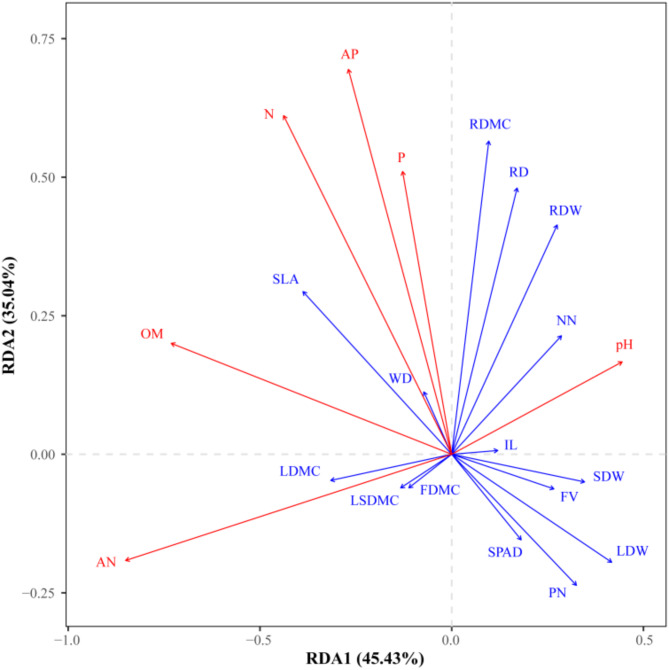
The relationship between soil factors and phenotypic traits of C. rhabdocladus was analyzed using RDA. The results showed that the eigenvalues on the first axis was 45.43% and 35.04% on the second axis, with the first two axes cumulatively explaining 80.47% of the variance in phenotypic traits (Fig. 4). This suggests that the effect of soil on phenotypic traits of C. rhabdocladus is well accounted for by the RDA model. As shown in the figure, soil total phosphorus content, total nitrogen content, and effective phosphorus content were positively correlated with root traits. Soil organic matter content was positively correlated with specific leaf area and negatively correlated with flagellum volume. Soil effective nitrogen content was positively correlated with leaf dry matter content, leaf sheath dry matter content, and flagellum dry matter content. Additionally, soil pH was positively correlated with the number of vine nodes.
Fig. 4.
Biplot of redundancy analysis of soil factors and phenotypic traits of C. rhabdocladus
The corrected R2 of the RDA model for soil factors and phenotypic traits was 0.204. the results of the covariance test showed that the variance inflation factor (VIF) values for all soil factors were below 10, indicating that there was no significant covariance among the soil factors (Table 5). The influence of soil pH, OM, nitrogen (N), and available nitrogen (AN) on the phenotypic traits of C. rhabdocladus was extremely significant (P < 0.001). The effect of soil P on C. rhabdocladus phenotype traits was highly significant (0.001 < P < 0.01), while the influence of available phosphorus (AP) was significant (0.01 < P < 0.05). Based on the R2 ranking of the importance of soil factors, AN > OM > pH > N > P > AP, indicating that AN and OM of the soil are the key factors influencing the phenotypic traits of C. rhabdocladus.
Table 5.
Redundancy analysis of soil factors and phenotypic traits
| Soil factor | VIF | F | P | R 2 | sort |
|---|---|---|---|---|---|
| pH | 1.843 | 7.013 | 0.001*** | 0.029 | 3 |
| OM | 8.240 | 9.140 | 0.001*** | 0.061 | 2 |
| N | 2.458 | 8.533 | 0.001*** | 0.025 | 4 |
| P | 2.476 | 5.709 | 0.002** | 0.013 | 5 |
| AN | 5.914 | 9.133 | 0.001*** | 0.068 | 1 |
| AP | 2.505 | 2.314 | 0.034* | 0.008 | 6 |
Variance decomposition analysis
The results of the variance decomposition based on the RDA of climate and soil factors on the phenotypic traits of C. rhabdocladus are shown in Fig. 5. The overall explained variance of environmental factors on the phenotypic traits of the C. rhabdocladus was 49.8%. Specifically, soil factors accounted for 4.1% of the phenotypic variation, the climate factor explained 31.4% of the phenotypic variation, and the combined effect of soil and climate together explained 14.8% of the phenotypic variation. These results indicate that the effect of climate on the phenotypic traits of C. rhabdocladus was more significant than that of soil.
Fig. 5.
Wayne plots of variance decomposition for climate and soil factors
Discussion
Variation and plasticity of phenotypic traits in the C. rhabdocladus
Plant traits are determined by genetic factors, environmental conditions, and their interactions [11]. In this study, the phenotypic traits of C. rhabdocladus among provenances showed high diversity, with a variation range of 7.48–182.16% observed among 15 traits. This high diversity of phenotypic traits among provenances of C. rhabdocladus may be attributed to heterogamous pollination, seed reproduction, wide distribution, and long-term geographic isolation [13].
Traits including leaf dry weight, number of pinnate leaves, number of nodes, internode length, stem dry weight, flagellum volume, root dry weight, root dry matter content, and root density exhibited a broad genetic base. In contrast, traits including leaf dry matter content, specific leaf area, leaf sheath dry matter content, chlorophyll SPAD value, wood density, and flagellum dry matter content displayed a narrow genetic base, making then less diverse. The coefficient of variation for 67.74% of the quantitative traits of Artocarpus heterophyllus were greater than 20%, which is consistent with the findings of this study [33]. Similarly, the low coefficient of variation for leaf dry matter of C. rhabdocladus was consistent with the low variability observed in the LDMC of Cunninghamia lanceolata at the provenance level. LDMC is closely related to plant growth rate and resource utilization strategies [13]. High coefficients of variation for several traits in Ceratonia siliqua strongly indicate the presence of significant phenotypic variation, confirming the clear differences between Ceratonia siliqua populations across different geographic regions [34].
Phenotypic plasticity is essential for enabling plant populations to respond to environmental changes in the short term and for maintaining genetic diversity over the long term. It serves as a key indicator of a plant’s ability to adapt to its environment [29, 35]. Lianas, For instance, lianas exhibit remarkable adaptability by adjusting the plasticity of morphological structures including leaves and stems, as well as biomass allocation in response to varying environmental conditions [36–38]. In this study, the plasticity indices of the phenotypic traits of C. rhabdocladus ranged from 0.16 to 0.81. The traits with the highest plasticity indices were the flagellum volume, stem weight, root weight, leaf weight, number of nodes, and number of plumes. This suggest that C. rhabdocladus adapts to different geographic environments by adjusting the plasticity in biomass allocation among its flagellum, roots, stems, and leaves. This phenotypic plasticity likely plays in the phenotypic traits is likely plays a key role in the wide distribution of the C. rhabdocladus. As a climbing organ, a large and robust flagellum enables C. rhabdocladus to maintain a competitive advantage over resources in the community. Supporting trees significantly influence vine trait plasticity, increasing stem biomass partitioning and decreasing root partitioning. Additionally, abiotic factors including soil and water availability also impact the phenotypic plasticity C. rhabdocladus vines [39]. In summary, the phenotypic trait plasticity of C. rhabdocladus provides the biological basis for its widespread distribution, while the biotic and abiotic environments within C. rhabdocladus communities shape its phenotypic variation and plasticity.
Correlation between phenotypic traits of C. rhabdocladus
Plant traits are not isolated; instead, they are intricately interconnected, with plant functional traits often adapting to specific environmental conditions through adjustment and combination of these traits [20]. In this study, we assessed multiple dimensions of phenotypic traits in C. rhabdocladus, including traits localized to leaves, leaf sheaths, stem segments, flagella, and roots, as well as whole-plant traits including the number of pinnate leaves and the number of nodes. The results revealed highly significant correlations among many of these traits in C. rhabdocladus.
For leaf traits, leaf dry weight represents the constant weight of a single leaf, which generally increases with increasing leaf area. Specific leaf area indicates the maximum extent to which the leaf can be expanded at a constant weight, and thus has a highly significant negative correlation with leaf dry weight. As leaves and leaf sheaths are closely connected, there is a significant positive correlation between leaf dry matter content and leaf sheath matter content. Specific leaf area was negatively correlated with the dry weight of a single leaf, but positively correlated with the total number of leaves of the whole plant. This suggests that both specific leaf area and the total number of leaves reflect the plant’s ability to utilize light energy. The number of pinnate leaves showed an extremely positive correlation with the chlorophyll SPAD value, which is also related to the plant’s ability to capture light [40, 41]. Light competition among plants is universal, and traits including large leaf area, high leaf numbers, and high chlorophyll content can help C. rhabdocladus form dense canopies that effectively intercept light [42]. Regarding leaf sheath traits, leaf sheath dry matter content was highly significantly negatively correlated with leaf dry weight, but highly significantly positively correlated with leaf dry matter content. Regarding stem traits, the stem serves as a critical organ for structural support and nutrient transport in C. rhabdocladus. Stem traits not only reflect the plant’s efficiency in transporting water and nutrients but also its ability to withstand external disturbances. Stem dry weight was highly significantly positively correlated with leaf dry weight, number of pinnate leaves, number of nodes, and flagellum volume, reflecting an important trade-off in growth rate. Wood density was also highly significantly positively correlated with leaf sheath dry matter content and flagellum dry matter content, indicating similar biomass allocation to stems, leaf sheaths, and flagella in C. rhabdocladus. The highly significant negative correlation between wood density and internode length suggests that as the internode length increases, wood density decreases, leading to a more sparsely organized structural characteristic. Regarding flagellum traits, the flagellum is an important climbing organ of the cane vine, and its traits are related to the ability to climb tall trees to capture light from high elevations. In this study, flagellum volume was highly significantly positively correlated with the dry weight of leaves, the number of pinnate leaves, the number of cane nodes, and the dry weight of stems. Flagellum dry matter content was highly significantly positively correlated with leaf dry matter content, leaf sheath dry matter content, and wood density, suggesting a convergence in the allocation of dry matter content across stems, leaf sheaths, and flagella in C. rhabdocladus. In terms of root traits, roots characterize the plant’s ability to absorb water and nutrients. Notably, the root traits were not significantly correlated with most of the aboveground organs, but they were positively correlated with the number of nodes. This finding suggests that taller plants have a more developed root system, and the number of nodes might be related to the age of the plant. Therefore, it is assumed that older plants may have more developed root systems [43].
In summary, there was a strong positive correlation among several leaf traits involved in light energy capture and nutrient production in C. rhabdocladus, with convergent trends observed in stems, leaf sheaths, and flagella. Root traits, however, were more independent and less strongly correlated with aboveground organs. There was a significant positive correlation between traits characterizing growth rate, as well as among traits characterizing survivorship. However, traits characterizing growth rate and traits characterizing survivorship showed a significant negative correlation, consistent with the principles of the “Leaf Economic Spectrum Theory” [44]. The correlations between traits have important implications for practical applications. For example, easily measurable traits including leaf dry weight can be used to assess the growth status of stems, sheaths whips, and other traits that are more challenging to measure, providing valuable assistance to breeders in selecting high-quality varieties. Overall, the correlations between phenotypic traits in C. rhabdocladus reflects its survival strategies in response to environmental changes.
Effect of climate factors on cane phenotypic traits
The geographic differentiation patterns of functional traits under different climatic conditions reflect differences in their growth strategies. Changes in temperature and moisture are the most common ecological constraints of plant production potential [45]. In this study, minimum temperature showed a significant positive correlation with wood density in C. rhabdocladus. This correlation is likely due to the influence of heat levels on the vine’s ability to absorb and transport nutrients and store energy [46]. Higher temperatures lead to an earlier onset cambial activity, accelerating cell division and resulting in the production of more wood cells, which increase C. rhabdocladus tissue density in areas with sufficient heat. This response is a unique adaptation strategy of lianas to tropical climates. This finding aligns with the positive correlation observed between wood density and temperature in Picea crassifolia [47], but contrasts with results of other tree species including Picea abies, for which wood density increases as temperatures decrease [45].
Minimum temperature was significantly negatively correlated with the number of rattan nodes, which were characterized by periodic changes in their growth and development. The number of rattan nodes was influenced by temperature rhythms, with prolonged periods of high temperature leading to a decrease in the number of nodes. This may represent an adaptive strategy in tropical environments [12]. In addition, average temperature, effective accumulated temperature, solar radiation intensity, and maximum temperature showed significant negative correlations with root traits. Low temperatures limit the size of eucalyptus leaves and may inhibit the growth of plant leaves, stems, and other organs [48]. Consequently, more resources need to be allocated to root development, which increases root volume and root density. This is an adaptive strategy of the C. rhabdocladus to survive in low-temperature environments. Rainfall showed a significant positive correlation with the number of pinnate leaves and chlorophyll SPAD values. In regions with high rainfall, light conditions may not always be optimal, and to adapt to low light conditions, C. rhabdocladus increases its investment in pinnate leaf number and chlorophyll content to capture more light resources, thereby optimizing its photosynthetic rate and light utilization [42]. The average air temperature represents the heat conditions of a region and has a significant impact on the morphology and resource allocation of rattan. It can explain the regional differences in the phenotypic traits of C. rhabdocladus [49]. Similar studies support the conclusions of this study; for example, average temperature significantly affected leaf size and leaf nutrient content in Cunninghamia lanceolata, with temperature showing a more significant impact on functional traits than precipitation [13]. Similarly, temperature was identified as the main driver affecting the functional traits of leaves in Dicranopteris pedata [20].
Effect of soil factors on phenotypic traits of C. rhabdocladus
Soil provides essential nutrients to plants, and soil physicochemical properties are closely related to plant traits [50]. Root, being in direct contact with soil, mainly perform the function of acquiring soil resources. Previous studies have shown that phosphorus is essential for promoting root development. In turn, root secretions can change local soil pH to make phosphorus more effective [43, 51]. Consequently, soil total phosphorus content and effective phosphorus content are closely related to the root traits of rattan. Within a given range, soil organic matter is an indication of soil fertility level, whereas specific leaf area can accurately represent plant adaptation to the environment, resource acquisition, and self-protection under intense light, and is closely related to photosynthesis and primary productivity [52]. In areas with high soil organic matter content and nutrient enrichment, C. rhabdocladus invests in greater specific leaf area to maximize photosynthetic rate, which facilitates resource acquisition and promotes plant growth and development. Interestingly, flagellum volume was negatively correlated with soil organic matter. As the climbing organ of C. rhabdocladus, the flagellum tends to degrade in fertile soils where the plant does not have to compete as intensely with other plants for light. In contrast, in poorer soils with limited below-ground nutrients, C. rhabdocladus relies more on energy obtained through photosynthesis, resulting in generally larger flagellum.
Soil nitrogen content is positively correlated with the dry matter content of leaves, leaf sheath, flagella, and other organs. In areas with high soil nitrogen content, higher dry matter content supports the construction of protection tissue structures in plants, which help resist adverse environment conditions and maintain their life span [53]. Soil pH affects the solubility and morphology of most nutrients in the soil, which in turn affects the plant nutrient absorption. For example, the effectiveness of phosphorus decreases in acidic soils, while the effectiveness of nitrogen and potassium reaches its peak in partially-neutral soils [11]. The number of cane nodes increases with soil pH within a certain range, likely due to the enhanced effectiveness of soil nutrients that promote the overall growth and development of the cane vine plant. The results of the RDA model analysis showed that soil AN and OM were the key soil factors influencing the phenotypic traits of C. rhabdocladus. On a large geographic scale, soil AN and OM can represent the soil fertility status in different regions and affect the growth and development of several organs of C. rhabdocladus.
Major factors explaining the pattern of geographic divergence in phenotypic traits of C. rhabdocladus
The variance decomposition analysis revealed that the effect of climate on the phenotypic traits of C. rhabdocladus was more significant than that of soil. On a larger scale, climate is the main macro-environmental filter influencing changes in plant phenotypic traits [21]. The pattern of variation in the phenotypic traits of C. rhabdocladus largely follows the gradient of climatic stability, resulting in a pattern of geographic divergence of these traits as a result of climate change. This study validates previous findings that plants were more significantly affected by climate at larger scales, with temperature and precipitation differences creating a filtering effect on phenotypic trait patterns of forest vegetation in different regions [21]. Climatic factors are important factors in determining ecosystem species composition and drive ecophysiological processes that influence the overall function and stability of the ecosystem. For instance, climatic characteristics significantly affect the radial growth and wood yield of Picea abies trunks [44]. Additionally, climate is the main factor explaining variation in plant seed size [54], and is the most important factor influencing variation in wood density among individuals within Pinus sylvestris populations [55].
The results of this study provide valuable insights for evaluating the germplasm of C. rhabdocladus from different geographical provenances and enhance our understanding of the species’ ecological strategies in adapting to various habitats under climate change. However, there are still limitations in this study. While it identifies the primary environmental factors influencing phenotypic variation in C. rhabdocladus, the specific mechanisms by which different environmental conditions affect plant responses remain unclear and require further investigation. In the future, path analysis can be used to determine the direct and indirect effects of climatic and soil factors on phenotypic traits. Additionally, the traits analyzed in this study are limited in number and scope, focusing primarily on easily measurable morphological traits and lacking biochemical indicators. In order to comprehensively evaluate the traits of C. rhabdocladus, biochemical traits including leaf nitrogen content should be tested in the future. The period of this study is relatively short, and it is only clear that the phenotypic traits change on the spatial scale, while it is uncertain whether the phenotypic traits maintain stable changes on the temporal scale. In the future, long-term continuous monitoring of the changes of traits and environment is needed. Seeds from different geographical sources could be collected and cultivated in the same environment, and long-term provenance tests can be conducted to accumulate rich and reliable experimental evidence. This would help clarify the stability of phenotypic traits in response to environmental factors. Previous studies have shown that species with a wide range of distribution are affected by geographical isolation, and gene flow is not smooth, and there may be different genotypes, so the phenotypic differentiation may be influenced by multiple factors including genes or environment. As for the sources of intraspecific variation of the phenotype of C. rhabdocladus, this study only provides environmental factors, and the genotypes of C. rhabdocladus from different geographical sources are still unknown. In the future, sequencing technology is needed to identify the genomic regions of related traits, and further studies are needed to distinguish the relationship between the three dimensions of heredity, soil and climate, and explore the main sources of phenotypic trait variation of C. rhabdocladus. In the future, with the help of molecular marker means - SNP, high-density genetic linkage map is drawn to determine the gene loci, which is conducive to more accurate and reliable mining of excellent germplasm and applied to the improvement of C. rhabdocladus varieties.
Conclusion
The phenotypic traits of C. rhabdocladus among provenances exhibited high variability and plasticity, with significant correlations among leaf, stem and thorn traits. Among the climatic factors, average temperature had the most substantial influence on phenotypic traits, while effective nitrogen content and organic matter content were the most significant soil factors. Overall, climate had a greater impact than soil on the phenotypic traits of C. rhabdocladus at a larger scale. These findings highlight the importance of climatic adaptation in shaping phenotypic diversity and suggest that future research should explore the genetic mechanisms underlying these traits and their interactions with environmental factors at finer spatial scales.
Acknowledgements
We extend our appreciation to Dr. Rui Gu and Jiarui Li for providing assistancet in plant material collection during the experimental phases of this study.
Author contributions
J N conducted the conceptualization, performed the methodology, carried out the investigation and wrote the original draft; R X contributed to the methodology, investigation and the writing of the original draft; S W contributed to the data curation; Y L contributed to the formal analysis; S F and Q Q had revised and improved the manuscript; G L carried out the investigation, acquired the funding and revised the manuscript. All authors read and agreed to the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2021YFD2200501), the Fundamental Research Funds for the International Centre for Bamboo and Rattan (1632021021), and the International Bamboo and Rattan Center Sanya Research Base Key Discipline Construction Project (YJPY2024001-2).
Data availability
All necessary data used to evaluate the conclusions in the manuscript are either included in the article itself or are readily available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This experiment did not involve endangered and protected species, and the collection of plant material complies with the guidelines and legislation of China. The collected materials were identified by Ruijing Xu, and the specimens were deposited in the International Center for Bamboo and Rattan Sanya Research Base.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junqing Nong and Ruijing Xu are co-first author.
References
- 1.Thomas HJD. Environmental drivers of plant form and function. Nat Ecol Evol. 2022;6:22–3. 10.1038/s41559-021-01576-z. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Sack L, Li Y, Zhang J, Yu K, Zhang Q, He N, Yu G. Relationships of stomatal morphology to the environment across plant communities. Nat Commun. 2023;14:6629. 10.1038/s41467-023-42136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporbert M, Jakubka D, Bucher SF, Hensen I, Freiberg M, Heubach K, König A, Nordt B, Plos C, Blinova I, Bonn A, Knickmann B, Koubek T, Linstädter A, Mašková T, Primack RB, Rosche C, Shah MA, Stevens AD, Tielbörger K, Träger S, Wirth C, Römermann C. Functional traits influence patterns in vegetative and reproductive plant phenology - a multi-botanical garden study. New Phytol. 2022;235:2199–210. 10.1111/nph.18345. [DOI] [PubMed] [Google Scholar]
- 4.Deng F, Xiao L, Huang J, Luo H, Zang R. Changes in leaf functional traits driven by environmental filtration in different monsoon tropical forest types. Forests. 2023;14:2101. 10.3390/f14102101. [Google Scholar]
- 5.Zhu J, Zhu H, Cao Y. Effect of simulated warming on leaf functional traits of urban greening plants. BMC Plant Biol. 2020;20:1–13. 10.1186/s12870-020-02359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Hou J, Xu L, Li M, Chen Z, Zhang Z, He N. Variation in functional trait diversity from tropical to cold-temperate forests and linkage to productivity. Ecol Indic. 2022;138:108864. 10.1016/j.ecolind.2022.108864. [Google Scholar]
- 7.Zhu M, Jiang L, Wu D, Li W, Yang H, He X. Effects of water control and nitrogen addition on functional traits and rhizosphere microbial community diversity of Haloxylon ammodendron seedlings. Forests. 2023;14(9):1879. 10.3390/f14091879. [Google Scholar]
- 8.Zarzosa PS, Herraiz AD, Olmo M, Ruiz-Benito P, Barrón V, Bastias CC, de la Riva EG, Villar R. Linking functional traits with tree growth and forest productivity in Quercus ilex forests along a Climatic gradient. Sci Total Environ. 2021;786147468. 10.1016/j.scitotenv.2021.147468. [DOI] [PubMed]
- 9.Helsen K, Acharya KP, Brunet J, Cousins SAO, Decocq G, Hermy M, Kolb A, Lemke IH, Lenoir J, Plue J, Verheyen K, Frenne PD, Graae BJ. Biotic and abiotic drivers of intraspecifc trait variation within plant populations of three herbaceous plant species along a latitudinal gradient. BMC Eco. 2017;1738. 10.1186/s12898-017-0151-y. [DOI] [PMC free article] [PubMed]
- 10.Hu X, Gao HY, Umer L, Guo M, Tan Y, Kang Q, Fang L, Shen Z, Xia K T. Strategy trade-off of predominant stress tolerance relative to competition and reproduction associated with plant functional traits under karst forests. Forests. 2023;14:1258. 10.3390/f14061258. [Google Scholar]
- 11.Zhang C, Zeng F, Zeng Z, Du H, Su L, Zhang L, Lu M, Zhang H. Impact of selected environmental factors on variation in leaf and branch traits on endangered karst Woody plants of Southwest China. Forests. 2022;13(7):1080. 10.3390/f13071080. [Google Scholar]
- 12.Sun Z, Prachanun N, Sonsuthi A, Chanthorn W, Brockelman WY, Nathalang A, Lin L, Bongers F. Whole-plant seedling functional traits suggest lianas also support fast-slow plant economics spectrum. Forests. 2022;13(7):990. 10.3390/f13070990. [Google Scholar]
- 13.Xu R, Cheng S, Zhou J, Tigabu M, Ma X, Li M. Intraspecifc variations in leaf functional traits of Cunninghamia lanceolata provenances. BMC Plant Biol. 2023;23:92. 10.1186/s12870-023-04097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R. The worldwide leaf economics spectrum. Nat. 2004;428(6985):821–7. 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Li Y, Bu H, et al. Responses of leaf traits of common broad-leaved Woody plants to environmental factors on the Eastern Qinghai-Xizang plateau. Chin J Plant Ecol. 2019;43(10):863–76. [Google Scholar]
- 16.Wang C, Lu J, Yao H, et al. Leaf functional traits of Abies georgei and their environmental response. J Forestry Environ. 2022;42(2):123–30. [Google Scholar]
- 17.Li J, Su X, Guo J, Xu W, Feng L, Wang T, Fu F, Wang G. Sex-related differences of ginkgo biloba in growth traits and wood properties. Forests. 2023;14(9):1809. 10.3390/f14091809. [Google Scholar]
- 18.Liu Q, Li J, Guo Z, Chang C, Wang H. The relationships between root traits and the soil erodibility of farmland shelterbelts in the Bashang region of China. Forests. 2023;14(9):1827. 10.3390/f14091827. [Google Scholar]
- 19.Yu X, Guan X, Xiao F, Zhang W, Yang Q, Wang Q, Wang S, Chen L. Effects of liming on the morphologies and nutrients of different functional fine roots of Cunninghamia lanceolata seedlings. Forest. 2022;13(6):822. 10.3390/f13060822. [Google Scholar]
- 20.Yu J, Li Q, Wu X, Zhu C, Huang S, Yang F, Hou X. Adaptational responses of leaf functional traits of Dicranopteris dichotoma to environmental factors in different vegetational restoration stages. Glob Ecol Conserv. 2023;44:e02484. 10.1016/j.gecco.2023.e02484. [Google Scholar]
- 21.Yao L, Xu Y, Wu C, Deng F, Yao L, Ai X, Zang R. Variation in the functional traits of forest vegetation along compound habitat gradients in different Climatic zones in China. Forests. 2023;14(6):1232. 10.3390/f14061232. [Google Scholar]
- 22.Manishimwe A, Ntirugulirwa B, Zibera E, Nyirambangutse B, Mujawamariya M, Dusenge ME, Bizuru E, Nsabimana D, Uddling J, Wallin G. Warming responses of leaf morphology are highly variable among tropical tree species. Forests. 2022;13(2):219. 10.3390/f13020219. [Google Scholar]
- 23.Yang Y, Huang Y, Wei W, et al. Changes of leaf traits along Climatic gradients of typical shrub grasses in Northwest arid region. Chin J Ecol. 2021. 10.13292/j.1000-4890.202112.002. [Google Scholar]
- 24.Zhao Y, Long W, Xiao C et al. (2024) Interannual responses of functional traits of plants in tropical cloud forests to environmental changes. Journal of Hainan University (Natural Science) https://kns.cnki.net/kcms/detail/46.1013.N.20230518.0912.004.html
- 25.Zhou H, Xu X, Jiang X, et al. Plant functional trait responses to dolomite and limestone karst forests in Southwest China. Forests. 2022;13(12):2187. 10.3390/f13122187. [Google Scholar]
- 26.Pei S, Chen S, Guo L, Henderson A, Raven P. Flora of China: Vol 23. Beijing: Science Press.
- 27.Yu X, Ji R, Li M, Xia X, Yin W, Liu C. Geographical variation in functional traits of leaves of Caryopteris mongholica and the role of climate. BMC Plant Biol. 2023;23:394. 10.1186/s12870-023-04410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poorter L, Castilho CV, Schietti J et al. (2018) Can traits predict individual growth performance? A test in a hyperdiverse tropical forest.new phytologist, 10.1111/nph.15206 [DOI] [PMC free article] [PubMed]
- 29.Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecol. 2000;81(7):1925–36. 10.1890/0012-9658(2000)081[1925:PPRTLO]2.0.CO;2. [Google Scholar]
- 30.Wang T, Wang X, Hu P, et al. Genetic diversity of phenotypic traits of 64 Agropyron germplasm resources. J Northwest A&F University (Nat Sci Ed). 2025;53(06):1–11. [Google Scholar]
- 31.Ou H, Dong L, Li J, et al. Relationship between phenotypic diversity of Phoebe Bournei deed traits and environmental geographical factors. J Northeast Forestry Univ. 2021;49(08):45–50. [Google Scholar]
- 32.Luo Z, Dang H, Liang L, et al. Characteristics and plasticity indexes of leaf functional traits during the natural regeneration of Phoebe Bournei. J Cent South Univ Forestry Technol. 2022;42(12):133–41. [Google Scholar]
- 33.Nakintu JAM, Albrecht C, Wangalwa R, Lejju JB, Olet EA. Morphological traits of jackfruit (Artocarpus heterophyllus Lam.): Indicators of diversity, selection and germplasm dispersion in Uganda. Sci Afr. 2023;22:e01900. 10.1016/j.sciaf.2023.e01900. [Google Scholar]
- 34.Kassout J, Hmimsa Y, Fatehi SE, Ouahrani AE, Kadaoui K, Chakkour S, Ariza-Mateos D, Palacios-Rodríguez G, Navarro-Cerrillo R, Ater M. Image analysis of Moroccan Carob seeds (Ceratonia siliqua L.) revealed substantial intraspecific variations depending on climate and geographic origin. Ecol Process. 2022;11:34. 10.1186/s13717-022-00378-w. [Google Scholar]
- 35.Matesanz S, Gianoli E, Valladares F. Global change and the evolution of phenotypic plasticity in plants. Ann Ny Acad Sci. 2010;1206(1):35–55. 10.1111/j.1749-6632.2010.05704.x. [DOI] [PubMed] [Google Scholar]
- 36.Cai Z, Poorter L, Cao K, Bongers F. Seedling growth strategies in Bauhinia species: comparing lianas and trees. Ann Botany. 2007;100(4):831–8. 10.1093/aob/mcm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avalos G, Mulkey SS. Photosynthetic and morphological acclimation of seedlings of tropical lianas to changes in the light environment. Am J Bot. 2014;101(12):2088–96. 10.3732/ajb.1400127. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, Yang D, Zhang YB, Liu Q, Wang YS, Ke Y, Xiao Y, Wang Q, Dossa GG, Schnitzer SA, Zhang J. Vessel dimorphism and wood traits in lianas and trees among three contrasting environments. Am J Bot. 2023;110(4):e16154. 10.1002/ajb2.16154. [DOI] [PubMed] [Google Scholar]
- 39.Wyka TP, Oleksyn J, Karolewski P, Schnitzer SA. Phenotypic correlates of the lianescent growth form: a review. Ann Botany. 2013;112(9):1667–81. 10.1093/aob/mct236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang D, Wang D, Ren Y, Qin Y, Wu L. Responses of leaf traits to submergence stress and analysis of the economic spectrum of plant species in an aquatic-terrestrial Ecotone, the Li river. Acta Ecol Sin. 2017;37:750–9. [Google Scholar]
- 41.Roa-Fuentes LL, Templer PH, Campo J. Effects of precipitation regime and soil nitrogen on leaf traits in seasonally dry tropical forests of the Yucatan Peninsula, Mexico. Oecologia. 2015;179:585–97. 10.1007/s00442-015-3354-y. [DOI] [PubMed] [Google Scholar]
- 42.Maire V, Wright IJ, Prentice IC, Batjes NH, Bhaskar R, van Bodegom PM, Cornwell WK, Ellsworth D, Niinemets Ü, Ordonez A, Reich PB, Santiago LS. Global effects of soil and climate on leaf photosynthetic traits and rates. Global Ecol Biogeogr. 2015;24:706–17. 10.1111/geb.12296. [Google Scholar]
- 43.Simpson AH, Richardson SJ, Laughlin DC. Soil-climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Global Ecol Biogeogr. 2016;25:8:964–78. 10.1111/geb.12457. [Google Scholar]
- 44.Wright IJ, Reich PB, Westoby M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol. 2001;15:423–34. 10.1046/j.0269-8463.2001.00542.x. [Google Scholar]
- 45.Leštianska A, Fleischer P Jr., Merganičová K, Fleischer P, Sr., Nalevanková P, Střelcová K. Effect of provenance and environmental factors on tree growth and tree water status of Norway Spruce. Forests. 2023;14(1):156. 10.3390/f14010156. [Google Scholar]
- 46.Gao H, Cui K, von Gadow K, Wang X. Using functional traits to improve estimates of height–diameter allometry in a temperate mixed forest. Forests. 2023;14(8):1604. 10.3390/f14081604. [Google Scholar]
- 47.Xu J, Lv J, Bao F, Huang R, Liu X, Robert E, Zhao Y. Response of wood density of Picea crassifolia to climate change in Qilian mountains of Northwestern China. J Beijing Forestry Univ. 2011;33:115–221. 10.13332/j.1000-1522.2011.05.004. [Google Scholar]
- 48.Liu H, Rutherford S, Wan JSH, Liu J, Zhang J, Afzal MR, Du D, Rossetto M. Variation in leaf functional and plant defense traits of introduced Eucalyptus species across environmental gradients in their new range in Southern China. Forests. 2023;14(5):936. 10.3390/f14050936. [Google Scholar]
- 49.Niinemets Ü. Global-scale Climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecol. 2001;82:453–69. 10.1890/0012-9658(2001)082. [0453:GSCCOL]2.0.CO;2. [Google Scholar]
- 50.Du H, Peng WX, Song TQ, Zeng FP, Wang KL, Song M, Zhang H. Spatial pattern of Woody plants and their environmental interpretation in the karst forest of Southwest China. Giornale Botanico Italiano. 2015;149:121–30. 10.1080/11263504.2013.796019. [Google Scholar]
- 51.Dong Y, Liu Y. Response of Korean Pine’s functional traits to geography and climate. PLoS ONE. 2017;12(9):e0184051. 10.1371/journal.pone.0184051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nam KJ, Lee EJ. Variation in leaf functional traits of the Korean maple (Acer pseudosieboldianum) along an elevational gradient in a montane forest in Southern Korea. J Ecol Environ. 2018;42:33. 10.1186/s41610-018-0096-x. [Google Scholar]
- 53.Xu R, Qiu Q, Nong J, Fan S, Liu G. Seasonal patterns and species variability in the leaf traits of dominant plants in the tropical rainforests of Hainan Island, China. Forests. 2023;14(3):522. 10.3390/f14030522. [Google Scholar]
- 54.Soper Gorden NL, Winkler KJ, Jahnke MR, Marshall E, Horky J, Hudelson Colton, Etterson JR. Geographic patterns of seed mass are associated with climate factors, but relationships vary between species. Am J Bot. 2016;103(1):60–72. 10.3732/ajb.1500295. [DOI] [PubMed] [Google Scholar]
- 55.Szaban J, Jelonek T, Okińczyc A, Kowalkowski W. Results of a 57-year-long research on variability of wood density of the Scots pine (Pinus sylvestris L.) from different provenances in Poland. Forests. 2023;14(3):480. 10.3390/f14030480. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All necessary data used to evaluate the conclusions in the manuscript are either included in the article itself or are readily available from the corresponding author upon reasonable request.