Abstract
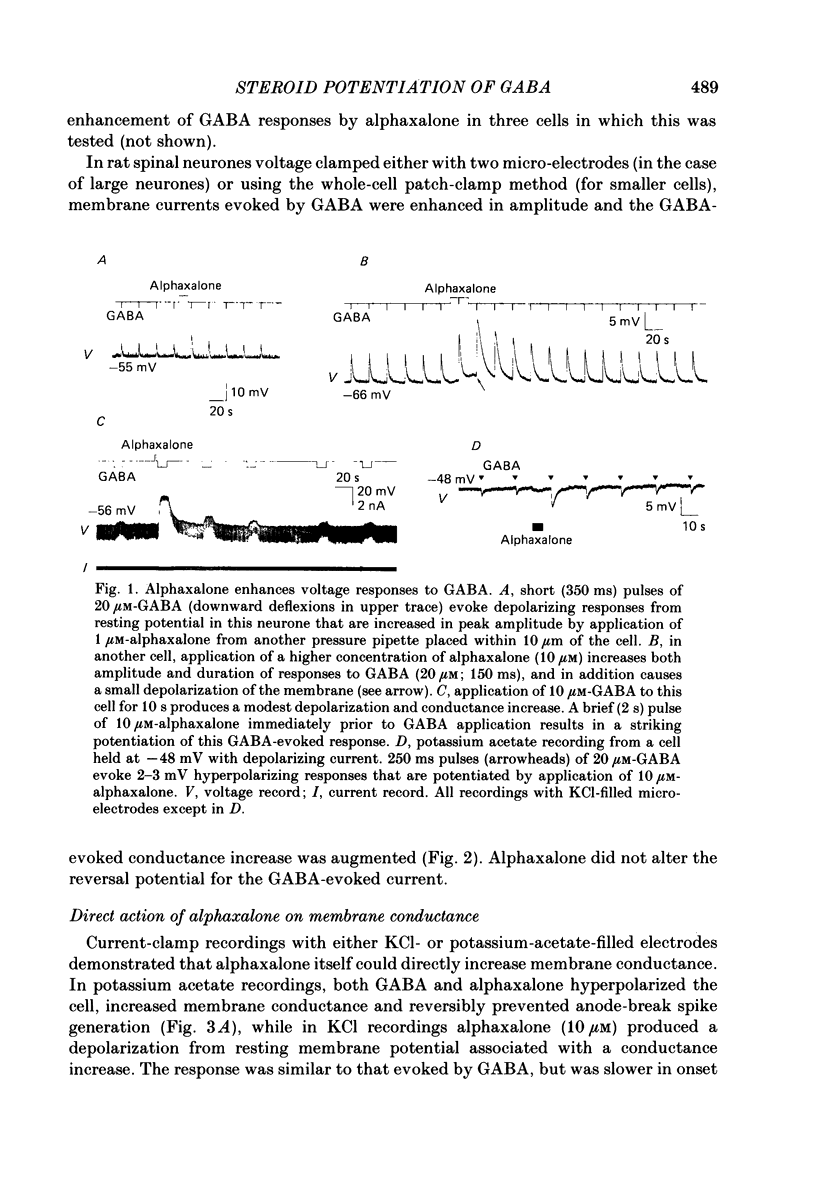
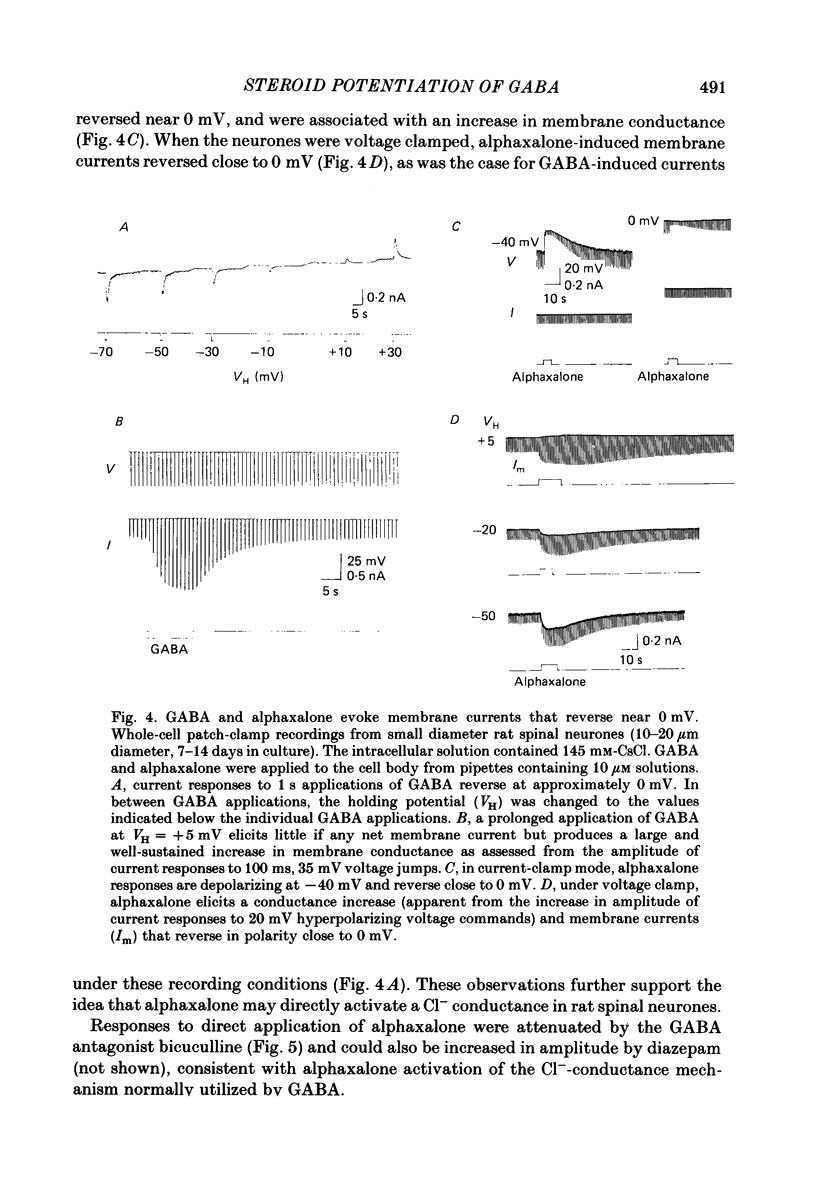
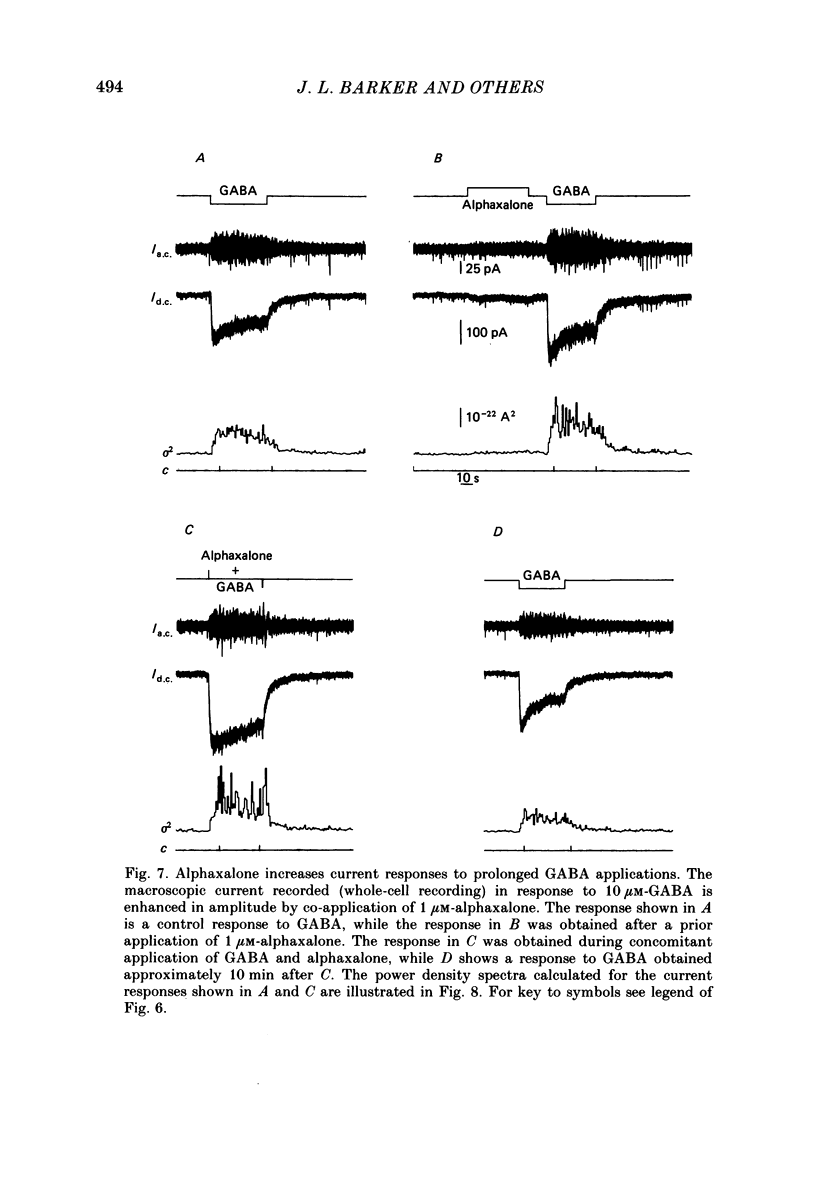
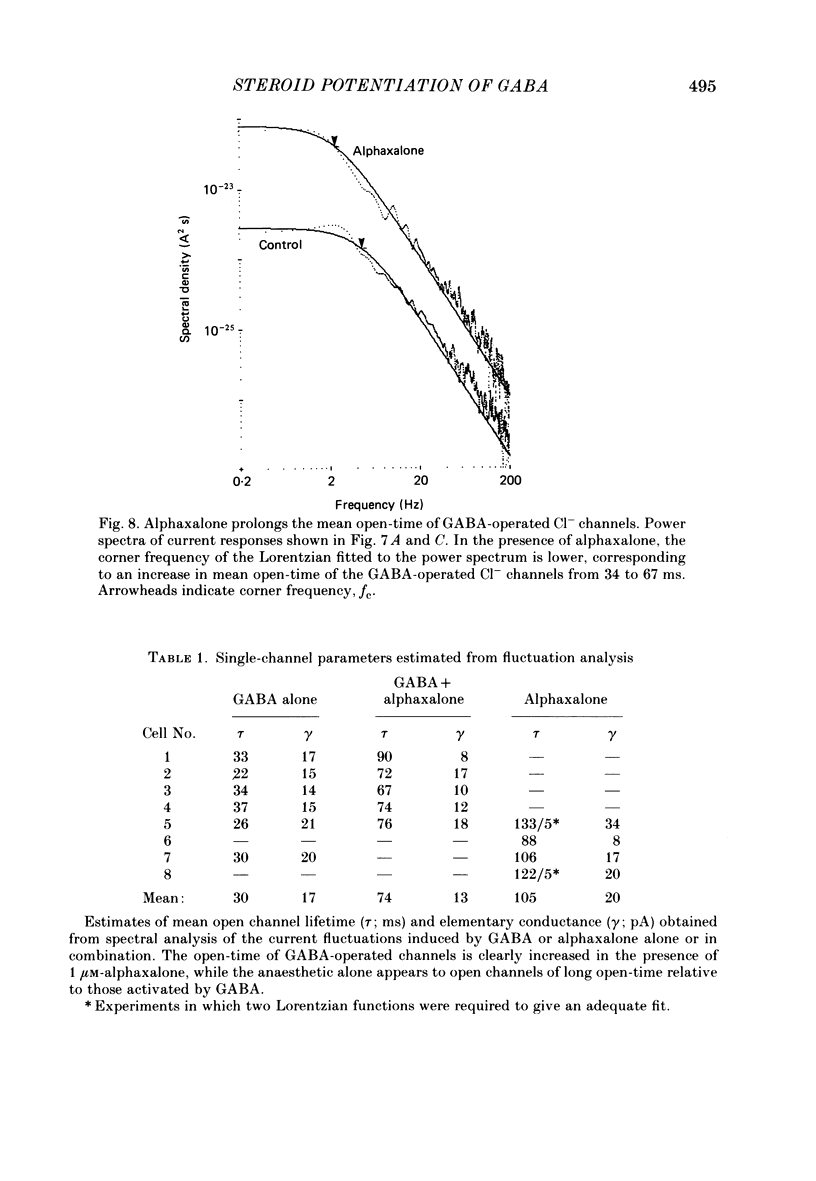
1. Intracellular recordings from cultured rat spinal cord neurones demonstrated that Cl(-)-dependent responses to GABA (gamma-aminobutyric acid) (but not glycine) were increased in amplitude and duration by the steroid anaesthetic alphaxalone (3 alpha-hydroxy-5 alpha-pregnane-11,20-dione) at submicromolar concentrations that produced little or no effect on passive electrical properties. The non-anaesthetic 3 beta-hydroxy analogue was without effect on GABA-evoked responses. 2. Under voltage clamp, membrane currents evoked by GABA were potentiated by alphaxalone without change in the reversal potential for the GABA-evoked response. Fluctuation analysis of GABA-evoked currents suggested that the mean open-time of GABA-activated channels was prolonged from 30 to 74 ms in the presence of the anaesthetic. 3. Higher concentrations of alphaxalone, similar to those reported during surgical anaesthesia, increased membrane conductance in the absence of exogenously applied GABA. Under voltage clamp, current responses to alphaxalone reversed at the same potential as did responses to GABA, suggesting that they result from increased Cl- conductance. 4. Alphaxalone responses were reduced by the GABA antagonist bicuculline. Fluctuation analysis of current responses to the anaesthetic suggest that they result from the activation of ion channels of long (100 ms) open-time and elementary conductance indistinguishable from that of channels activated by GABA (20 pS). Taken together, these findings indicate that the steroid anaesthetic is able to directly activate Cl- conductance normally activated by GABA in spinal neurones. 5. The actions of the steroid at GABA-receptor-Cl(-)-channel complexes are similar to those produced by the anaesthetic barbiturates (e.g. pentobarbitone), although obtained at 50-100-fold lower concentrations. These effects on the inhibitory Cl(-)-conductance mechanism may be partly responsible for the depressant actions of alphaxalone on the mammalian central nervous system.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Davies M. F., Blaxter T. J., O'Beirne M. Enhanced neuronal K+ conductance: a possible common mechanism for sedative-hypnotic drug action. Can J Physiol Pharmacol. 1985 Jul;63(7):831–837. doi: 10.1139/y85-137. [DOI] [PubMed] [Google Scholar]
- Child K. J., Currie J. P., Dis B., Dodds M. G., Pearce D. R., Twissell D. J. The pharmacological properties in animals of CT1341--a new steroid anaesthetic agent. Br J Anaesth. 1971 Jan;43(1):2–13. doi: 10.1093/bja/43.1.2-a. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillo B., Lass Y. The mechanism of steroid anaesthetic (alphaxalone) block of acetylcholine-induced ionic currents. Br J Pharmacol. 1984 Aug;82(4):783–789. doi: 10.1111/j.1476-5381.1984.tb16474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984 Dec 10;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Barbiturate reduction of calcium-dependent action potentials: correlation with anesthetic action. Brain Res. 1982 Mar 18;236(1):157–171. doi: 10.1016/0006-8993(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Mathers D. A., Barker J. L. Single channel currents activated by gamma-aminobutyric acid, muscimol, and (-)-pentobarbital in cultured mouse spinal neurons. J Neurosci. 1982 Jul;2(7):889–894. doi: 10.1523/JNEUROSCI.02-07-00889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. K., Gill E. W. Structurally specific effects of some steroid anesthetics on spin-labeled liposomes. Mol Pharmacol. 1975 May;11(3):280–286. [PubMed] [Google Scholar]
- Lodge D., Anis N. A. Effects of ketamine and three other anaesthetics on spinal reflexes and inhibitions in the cat. Br J Anaesth. 1984 Oct;56(10):1143–1151. doi: 10.1093/bja/56.10.1143. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. (-)Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science. 1980 Jul 25;209(4455):507–509. doi: 10.1126/science.6248961. [DOI] [PubMed] [Google Scholar]
- Mathers D. A. Spontaneous and GABA-induced single channel currents in cultured murine spinal cord neurons. Can J Physiol Pharmacol. 1985 Oct;63(10):1228–1233. doi: 10.1139/y85-203. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Presynaptic action of barbiturates in the frog spinal cord. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1460–1463. doi: 10.1073/pnas.72.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Snowman A. M. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J Neurosci. 1982 Dec;2(12):1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Yuzaki M. Patch-clamp studies of chloride channels activated by gamma-aminobutyric acid in cultured hippocampal neurones of the rat. Neurosci Res. 1984 Oct;1(5):275–293. doi: 10.1016/0168-0102(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Hamill O. P., Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J Neural Transm Suppl. 1983;18:83–95. [PubMed] [Google Scholar]
- Scholfield C. N. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980 Feb;383(3):249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Schulz D. W., Macdonald R. L. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res. 1981 Mar 23;209(1):177–188. doi: 10.1016/0006-8993(81)91179-3. [DOI] [PubMed] [Google Scholar]
- Sear J. W., Prys-Roberts C. Plasma concentrations of alphaxalone during continuous infusion of Althesin. Br J Anaesth. 1979 Sep;51(9):861–865. doi: 10.1093/bja/51.9.861. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Jr, Barker J. L., Smith B. M., Colburn T. R. Voltage clamping with microelectrodes. J Neurosci Methods. 1980 Dec;3(2):105–128. doi: 10.1016/0165-0270(80)90020-5. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Barbiturates decrease voltage-dependent calcium conductance of mouse neurons in dissociated cell culture. Mol Pharmacol. 1985 Sep;28(3):269–277. [PubMed] [Google Scholar]



