Abstract
1. The input from cutaneous nociceptors to climbing fibres projecting to the forelimb area of the C3 zone in the cerebellar anterior lobe was examined in barbiturate-anaesthetized cats. Climbing fibre responses were simultaneously recorded in single Purkinje cells and as field potentials from the cerebellar surface close to these cells. 2. The cutaneous receptive field of the climbing fibres studied were located on the ipsilateral forelimb. All climbing fibres were activated by both non-noxious tactile stimulation and noxious pinch of the skin. The location of the receptive field and the distribution of sensitivity in the receptive field appeared to be identical for noxious and tactile stimuli. 3. A phasic response in the climbing fibres was evoked by either short- or long-lasting non-noxious pressure applied to their cutaneous receptive field. By contrast, all climbing fibres studied were strongly and tonically activated (up to 4-11 Hz for the duration of the stimulation) by sustained noxious pinch in the most sensitive area of their receptive fields. 4. Experiments with anodal block of impulse conduction in myelinated fibres indicated that a major input to climbing fibres during sustained noxious pinch originates from nociceptive C fibres. 5. Sustained noxious pinch of the skin evoked large field potentials on the cerebellar surface. These field potentials were evoked simultaneously with climbing fibre responses in single Purkinje cells and were due to synchronous activation of many climbing fibres. These field potentials and discharges in single climbing fibres were elicited from the same area of the skin suggesting that many of the synchronously discharging climbing fibres have the same receptive field on the skin.
Full text
PDF

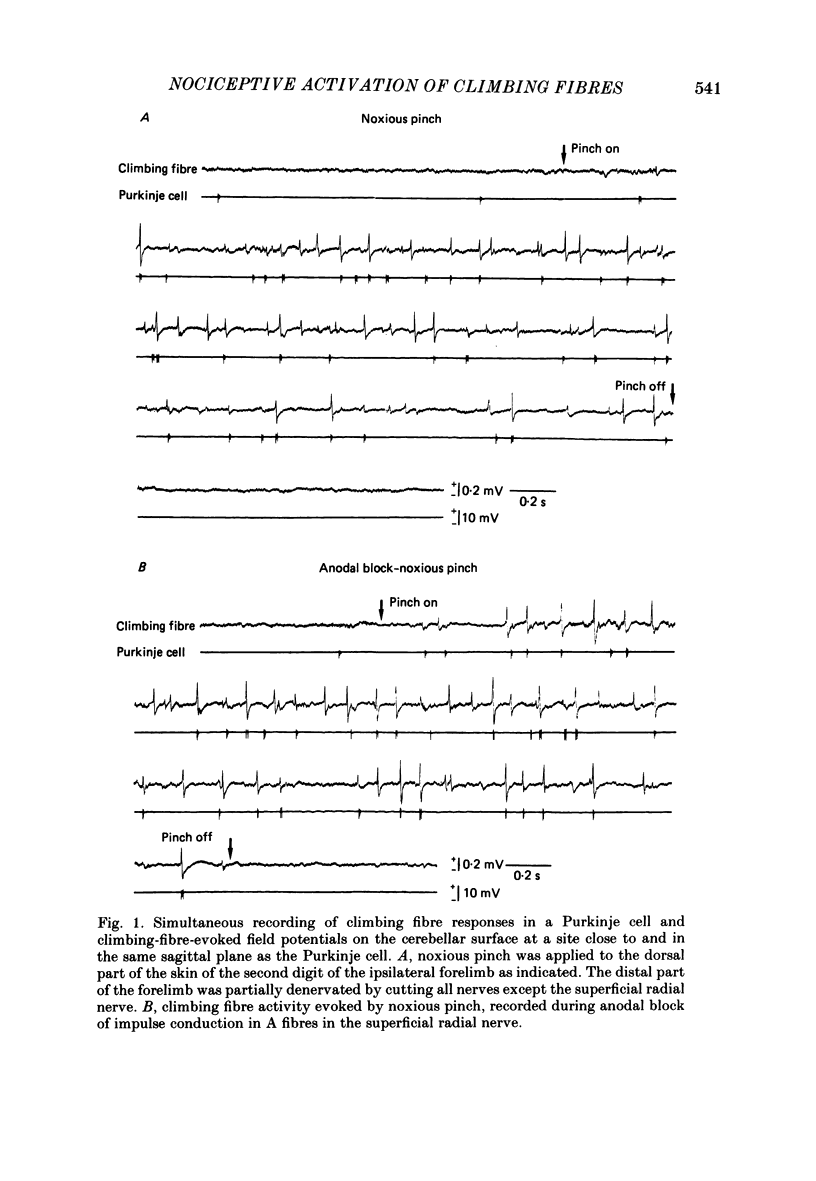

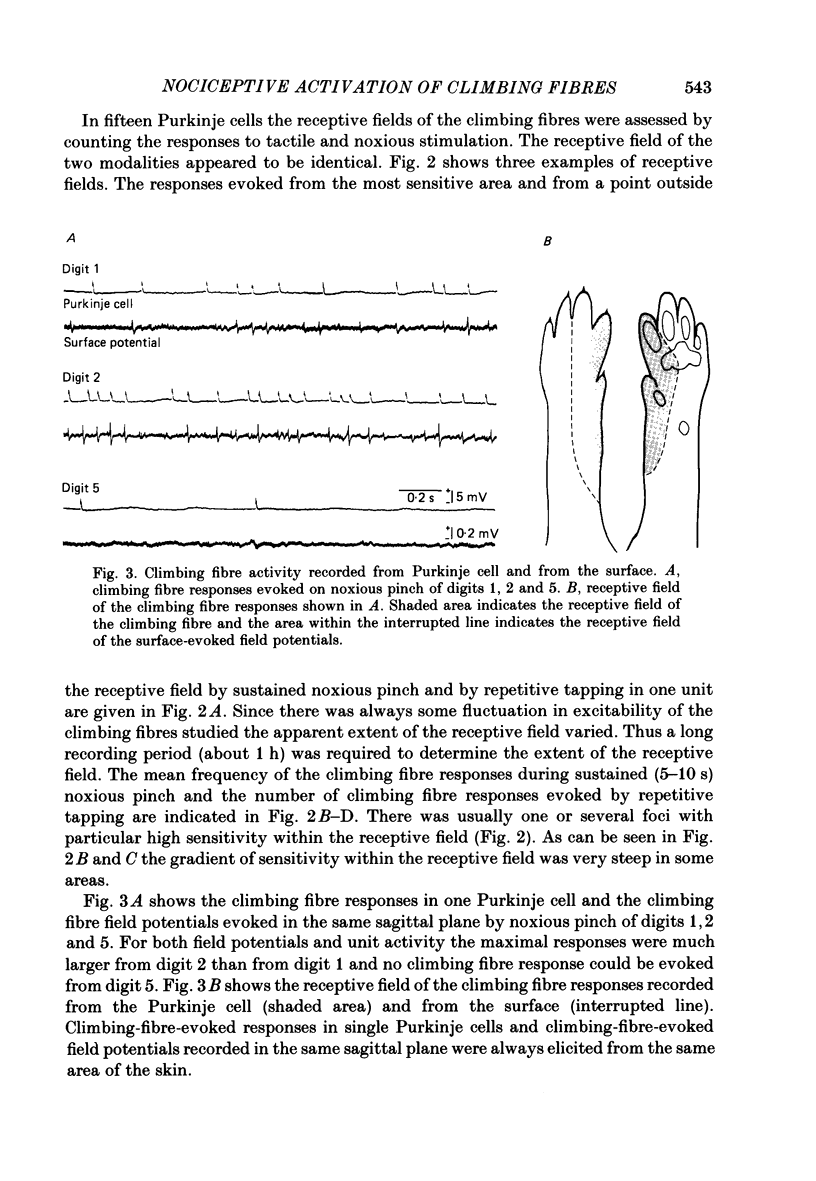



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Nyquist J. Origin and sagittal termination areas of cerebro-cerebellar climbing fibre paths in the cat. J Physiol. 1983 Apr;337:257–285. doi: 10.1113/jphysiol.1983.sp014623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Bessou P., Burgess P. R., Perl E. R., Taylor C. B. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971 Jan;34(1):116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. 3. In Purkyne cells by climbing fiber input. Exp Brain Res. 1972 Oct 29;15(5):484–497. doi: 10.1007/BF00236404. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Gustavsson P., Oscarsson O., Schouenborg J. Climbing fibres projecting to cat cerebellar anterior lobe activated by cutaneous A and C fibres. J Physiol. 1987 May;386:529–538. doi: 10.1113/jphysiol.1987.sp016549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985 Sep 9;342(2):357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- Gellman R., Gibson A. R., Houk J. C. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985 Jul;54(1):40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- IGGO A. Cutaneous heat and cold receptors with slowly conducting (C) afferent fibres. Q J Exp Physiol Cogn Med Sci. 1959 Oct;44:362–370. doi: 10.1113/expphysiol.1959.sp001417. [DOI] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972 May 12;40(1):81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- Ito M., Sakurai M., Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982 Mar;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht R., Rowe M. J., Schmidt R. F. Cutaneous convergence on to the climbing fibre input to cerebellar Purkyne cells. J Physiol. 1973 Feb;228(3):601–618. doi: 10.1113/jphysiol.1973.sp010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Baker R., Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974 May;37(3):560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Llinás R., Volkind R. A. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973 Aug 31;18(1):69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Rawson J. A., Tilokskulchai K. Suppression of simple spike discharges of cerebellar Purkinje cells by impulses in climbing fibre afferents. Neurosci Lett. 1981 Sep 1;25(2):125–130. doi: 10.1016/0304-3940(81)90319-0. [DOI] [PubMed] [Google Scholar]
- Rushmer D. S., Roberts W. J., Augter G. K. Climbing fiber responses of cerebellar Purkinje cells to passive movement of the cat forepaw. Brain Res. 1976 Apr 16;106(1):1–20. doi: 10.1016/0006-8993(76)90069-x. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C., Llinas R., Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974 May;37(3):541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]




