Abstract
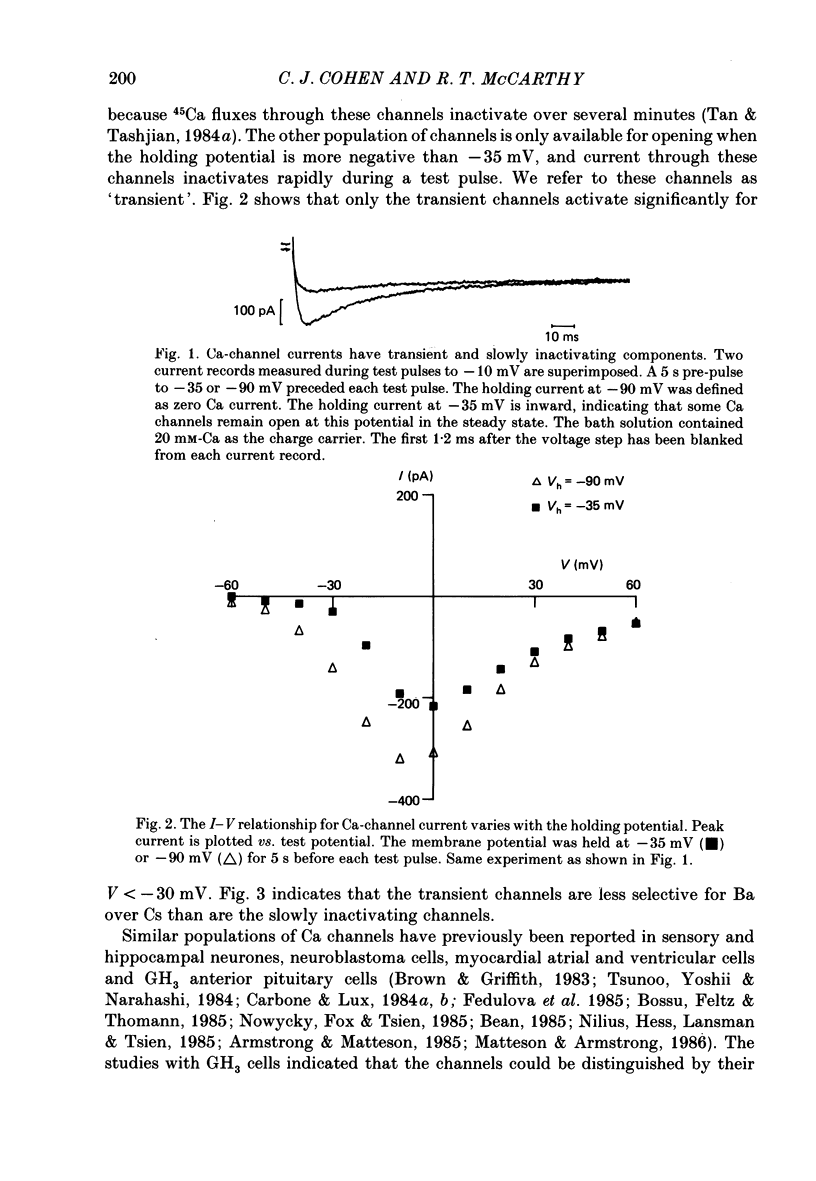
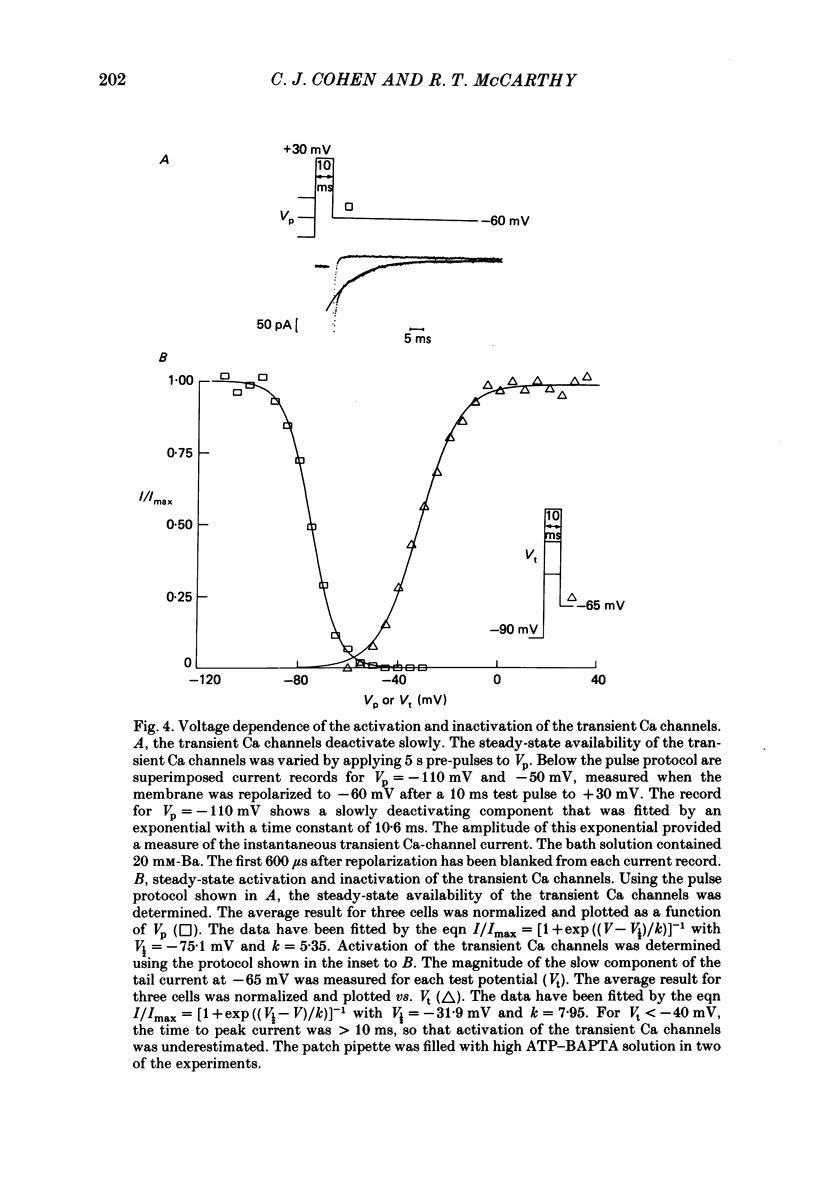
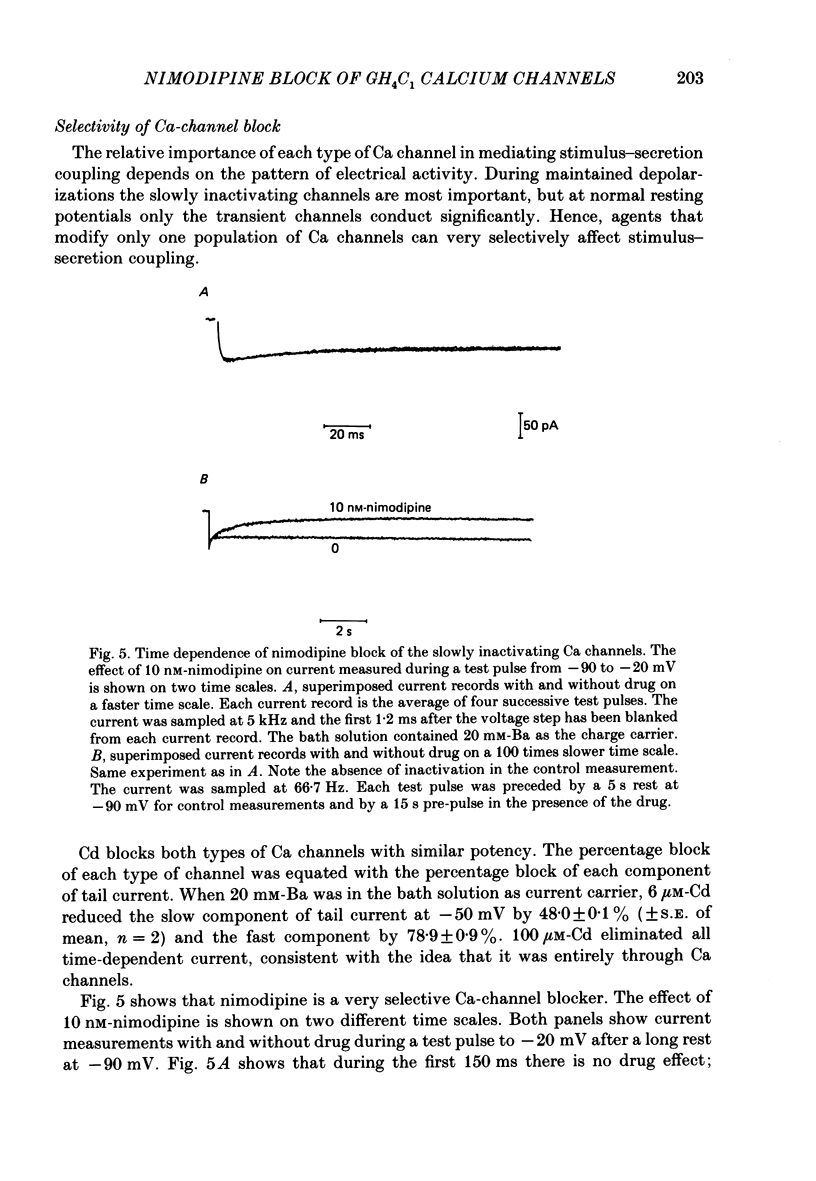
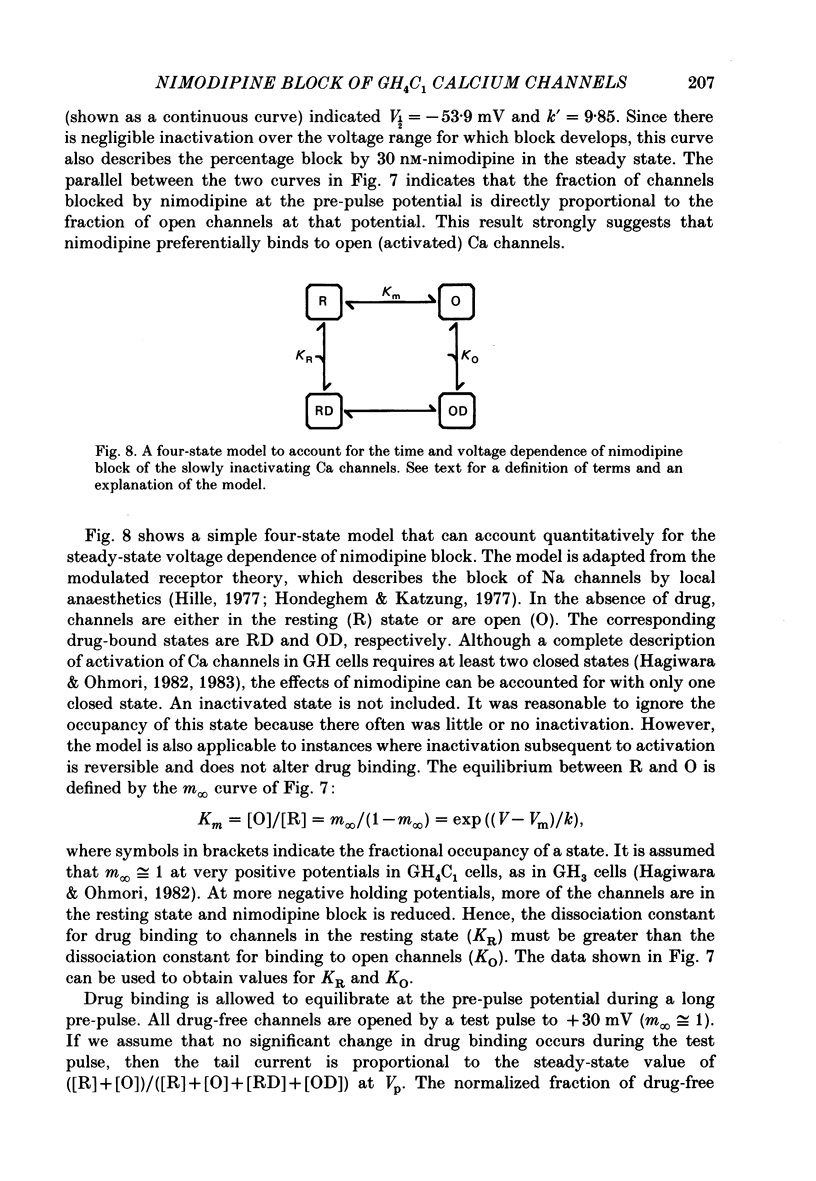
1. Ca channels were studied in the GH4C1 clonal cell line derived from rat anterior pituitary cells. The whole-cell variation of the patch-electrode voltage-clamp technique was used. 2. Two types of Ca channels were found. One type ('slowly inactivating' channels) is insensitive to changes in holding potential, does not inactivate during test pulses lasting several seconds, and deactivates very quickly upon repolarization. For holding potentials less than -40 mV, a second type of Ca channel is available for opening. This population ('transient' channels) differs from the first type in that it activates at more negative potentials, inactivates rapidly with either Ca or Ba as the charge carrier, deactivates about 10 times more slowly upon repolarization, and is less selective for Ba over Cs. 3. Nimodipine preferentially blocks the slowly inactivating channels. Block of these channels is time- and voltage-dependent, such that block is maximized by long depolarizations. 4. A comparison of the voltage dependence of steady-state nimodipine block with the voltage dependence of channel activation indicates that channel block is directly proportional to the number of open channels. The results are accounted for by a model that postulates 1:1 high-affinity drug binding to open Ca channels. The apparent dissociation constant for binding to open channels is 517 pM. Similar binding constants were previously reported for the inhibition of high-K-induced hormone secretion and high-affinity ligand binding of [3H]nimodipine to isolated plasma membranes. 5. The rate of onset of nimodipine block increases with the test potential, in quantitative agreement with the model of open-channel block. The apparent association rate is about 9.6 X 10(7) M-1 s-1; the dissociation rate is about 0.050 s-1. At therapeutic concentrations (less than 10 nM) nimodipine block takes many seconds to reach equilibrium. 6. Nimodipine should have little effect on stimulus-secretion coupling in healthy pituitary cells in vivo because: (a) the drug binds very weakly to the transient channels that are open at normal resting potentials, and (b) negligible high-affinity binding occurs during spontaneous activity because the onset of block is very slow.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abadie E., Gauville C., Brisson C. Nifedipine and endocrine status in diabetic patients. Br J Clin Pharmacol. 1984 Oct;18(4):648–650. doi: 10.1111/j.1365-2125.1984.tb02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Relationship of thyrotropin-releasing hormone-induced spike and plateau phases in cytosolic free Ca2+ concentrations to hormone secretion. Selective blockade using ionomycin and nifedipine. J Biol Chem. 1984 Dec 25;259(24):15350–15363. [PubMed] [Google Scholar]
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Dufy B., Owen D. G., Segal M. Excitable membrane properties of cultured central nervous system neurons and clonal pituitary cells. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):259–268. doi: 10.1101/sqb.1983.048.01.028. [DOI] [PubMed] [Google Scholar]
- Barros F., Katz G. M., Kaczorowski G. J., Vandlen R. L., Reuben J. P. Calcium currents in GH3 cultured pituitary cells under whole-cell voltage-clamp: inhibition by voltage-dependent potassium currents. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1108–1112. doi: 10.1073/pnas.82.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Boll W., Lux H. D. Action of organic antagonists on neuronal calcium currents. Neurosci Lett. 1985 May 23;56(3):335–339. doi: 10.1016/0304-3940(85)90265-4. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Persistent slow inward calcium current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:303–320. doi: 10.1113/jphysiol.1983.sp014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., Seay S. G. Structure-function relationship of calcium ion channel antagonists at the pituitary gonadotrope. Endocrinology. 1983 Nov;113(5):1592–1595. doi: 10.1210/endo-113-5-1592. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Slowing effects of dopamine and calcium-channel blockers on frequency of sodium spikes in rat pars intermedia cells. J Physiol. 1982 May;326:201–211. doi: 10.1113/jphysiol.1982.sp014186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Dual modulation of K channels by thyrotropin-releasing hormone in clonal pituitary cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4282–4286. doi: 10.1073/pnas.82.12.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B., Vincent J. D., Fleury H., Du Pasquier P., Gourdji D., Tixier-Vidal A. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979 May 4;204(4392):509–511. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Aizawa T., Hinkle P. M. Dihydropyridine Ca2+ antagonists: potent inhibitors of secretion from normal and transformed pituitary cells. Am J Physiol. 1985 May;248(5 Pt 1):C510–C519. doi: 10.1152/ajpcell.1985.248.5.C510. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Gurney A. M., Nerbonne J. M., Lester H. A. Photoinduced removal of nifedipine reveals mechanisms of calcium antagonist action on single heart cells. J Gen Physiol. 1985 Sep;86(3):353–379. doi: 10.1085/jgp.86.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of single calcium channel currents in rat clonal pituitary cells. J Physiol. 1983 Mar;336:649–661. doi: 10.1113/jphysiol.1983.sp014603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Isles T. E., Baty J., Dow R. J. The effect of nicardipine on pituitary hormone release in normal volunteers. Br J Clin Pharmacol. 1985;20 (Suppl 1):84S–87S. doi: 10.1111/j.1365-2125.1985.tb05147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski G. J., Vandlen R. L., Katz G. M., Reuben J. P. Regulation of excitation-secretion coupling by thyrotropin-releasing hormone (TRH): evidence for TRH receptor-ion channel coupling in cultured pituitary cells. J Membr Biol. 1983;71(1-2):109–118. doi: 10.1007/BF01870679. [DOI] [PubMed] [Google Scholar]
- Kanaya S., Arlock P., Katzung B. G., Hondeghem L. M. Diltiazem and verapamil preferentially block inactivated cardiac calcium channels. J Mol Cell Cardiol. 1983 Feb;15(2):145–148. doi: 10.1016/0022-2828(83)90291-2. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K., Kojima I., Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984 Nov;247(5 Pt 1):E645–E650. doi: 10.1152/ajpendo.1984.247.5.E645. [DOI] [PubMed] [Google Scholar]
- Krol G. J., Noe A. J., Yeh S. C., Raemsch K. D. Gas and liquid chromatographic analyses of nimodipine calcium antagonist in blood plasma and cerebrospinal fluid. J Chromatogr. 1984 Jan 13;305(1):105–118. doi: 10.1016/s0378-4347(00)83318-6. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A. Calcium-antagonists and islet function-XII. Comparison between nifedipine and chemically related drugs. Biochem Pharmacol. 1981 May 15;30(10):1039–1041. doi: 10.1016/0006-2952(81)90439-1. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. A., Struthers A. D. Calcium antagonists and hormone release. Clin Sci (Lond) 1984 Mar;66(3):249–255. doi: 10.1042/cs0660249. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Freedman S. B. Are dihydropyridine binding sites voltage sensitive calcium channels? Life Sci. 1984 Mar 26;34(13):1205–1221. doi: 10.1016/0024-3205(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz W., Stämpfli R. Block of Na channels in the membrane of myelinated nerve by benzocaine. Pflugers Arch. 1981 Jun;390(3):230–236. doi: 10.1007/BF00658267. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nishi K., Oyama Y. Accelerating effects of pentobarbitone on the inactivation process of the calcium current in Helix neurones. Br J Pharmacol. 1983 Jul;79(3):645–654. doi: 10.1111/j.1476-5381.1983.tb10001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electric activity of rat anterior pituitary cells in vitro. Acta Physiol Scand. 1978 Mar;102(3):330–341. doi: 10.1111/j.1748-1716.1978.tb06080.x. [DOI] [PubMed] [Google Scholar]
- Rae J. L., Levis R. A. Patch Clamp Recordings from the Epithelium of the Lens Obtained using Glasses Selected for Low Noise and Improved Sealing Properties. Biophys J. 1984 Jan;45(1):144–146. doi: 10.1016/S0006-3495(84)84142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F., Kelly R. B. A molecular description of nerve terminal function. Annu Rev Biochem. 1983;52:871–926. doi: 10.1146/annurev.bi.52.070183.004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. G., Sarmiento J. G., Herbette L. G. Kinetics of binding of membrane-active drugs to receptor sites. Diffusion-limited rates for a membrane bilayer approach of 1,4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985 Jun;27(6):612–623. [PubMed] [Google Scholar]
- Ronning S. A., Heatley G. A., Martin T. F. Thyrotropin-releasing hormone mobilizes Ca2+ from endoplasmic reticulum and mitochondria of GH3 pituitary cells: characterization of cellular Ca2+ pools by a method based on digitonin permeabilization. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6294–6298. doi: 10.1073/pnas.79.20.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Shangold G. A., Kongsamut S., Miller R. J. Characterization of voltage-sensitive calcium channels in a clonal pituitary cell line. Life Sci. 1985 Jun 10;36(23):2209–2215. doi: 10.1016/0024-3205(85)90331-5. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Changes in cytosolic ionized calcium induced by activators of secretion in GH3 cells. Am J Physiol. 1984 Feb;246(2 Pt 1):E198–E201. doi: 10.1152/ajpendo.1984.246.2.E198. [DOI] [PubMed] [Google Scholar]
- Struthers A. D., Millar J. A., Beastall G. H., McIntosh W. B., Reid J. L. Calcium antagonists and hormone release: effect of nifedipine on luteinizing hormone-releasing hormone and thyrotropin-releasing hormone-induced pituitary hormone release. J Clin Endocrinol Metab. 1983 Feb;56(2):401–404. doi: 10.1210/jcem-56-2-401. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. I. Characterization by 45Ca2+ fluxes. J Biol Chem. 1984 Jan 10;259(1):418–426. [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. II. Participation in thyrotropin-releasing hormone action on prolactin release. J Biol Chem. 1984 Jan 10;259(1):427–434. [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Electrical activity in adenohypophyseal cells and effects of hypophyseotropic substances. Fed Proc. 1984 Jun;43(9):2373–2378. [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Electrical behaviour in a line of anterior pituitary cells (GH cells) and the influence of the hypothalamic peptide, thyrotrophin releasing factor. Neuroscience. 1980;5(2):421–431. doi: 10.1016/0306-4522(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Titeler M., De Souza E. B., Kuhar M. J. [3H]Nitrendipine binding to calcium channels in bovine and rat pituitary. J Neurochem. 1985 Jun;44(6):1955–1958. doi: 10.1111/j.1471-4159.1985.tb07194.x. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Goldin S. M. Calcium channels in rat brain synaptosomes: identification and pharmacological characterization. High affinity blockade by organic Ca2+ channel blockers. J Neurosci. 1985 Mar;5(3):841–849. doi: 10.1523/JNEUROSCI.05-03-00841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J Gen Physiol. 1977 Mar;69(3):293–323. doi: 10.1085/jgp.69.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]


