Abstract
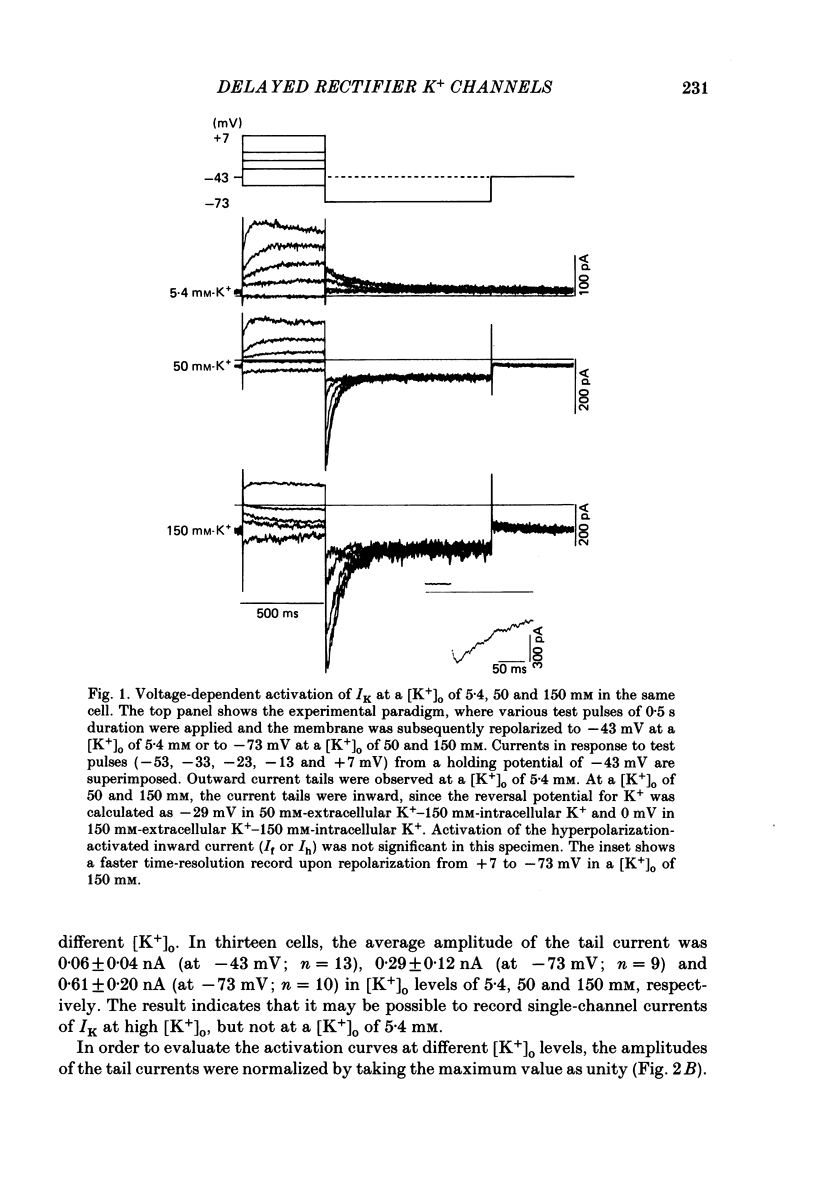
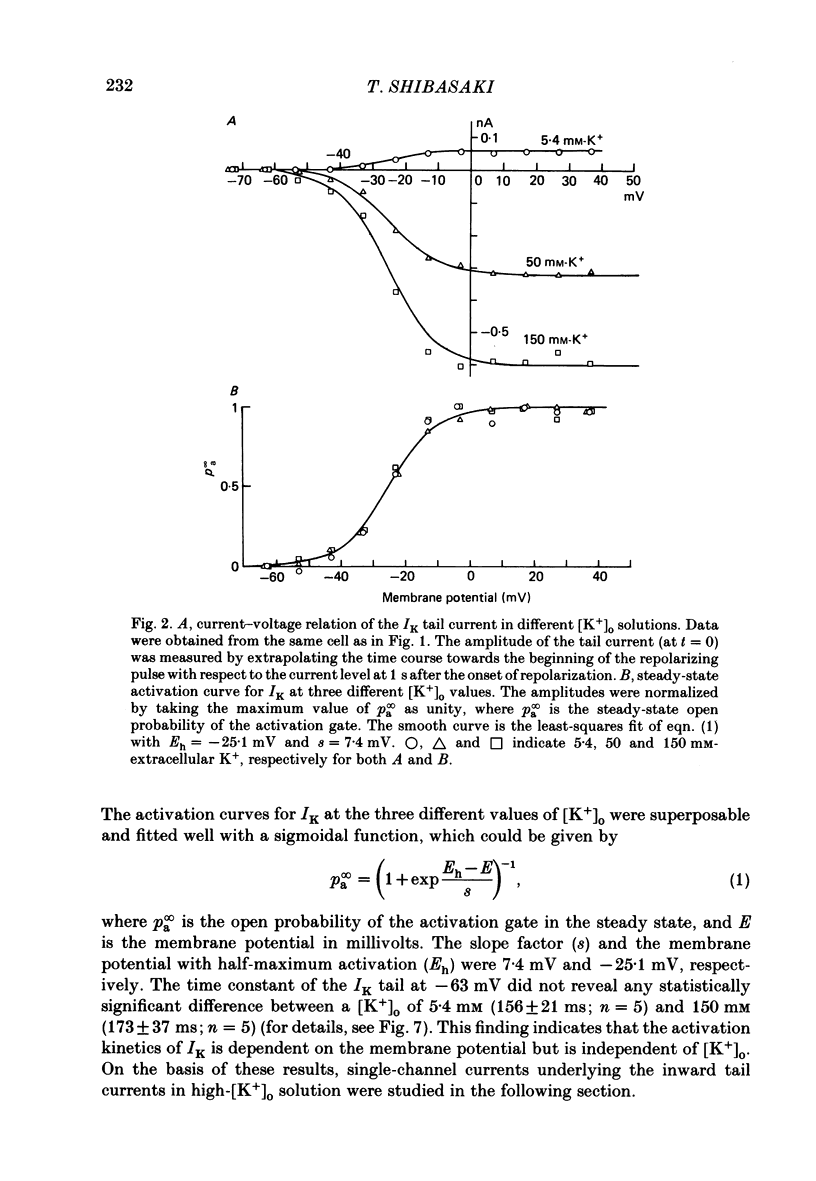
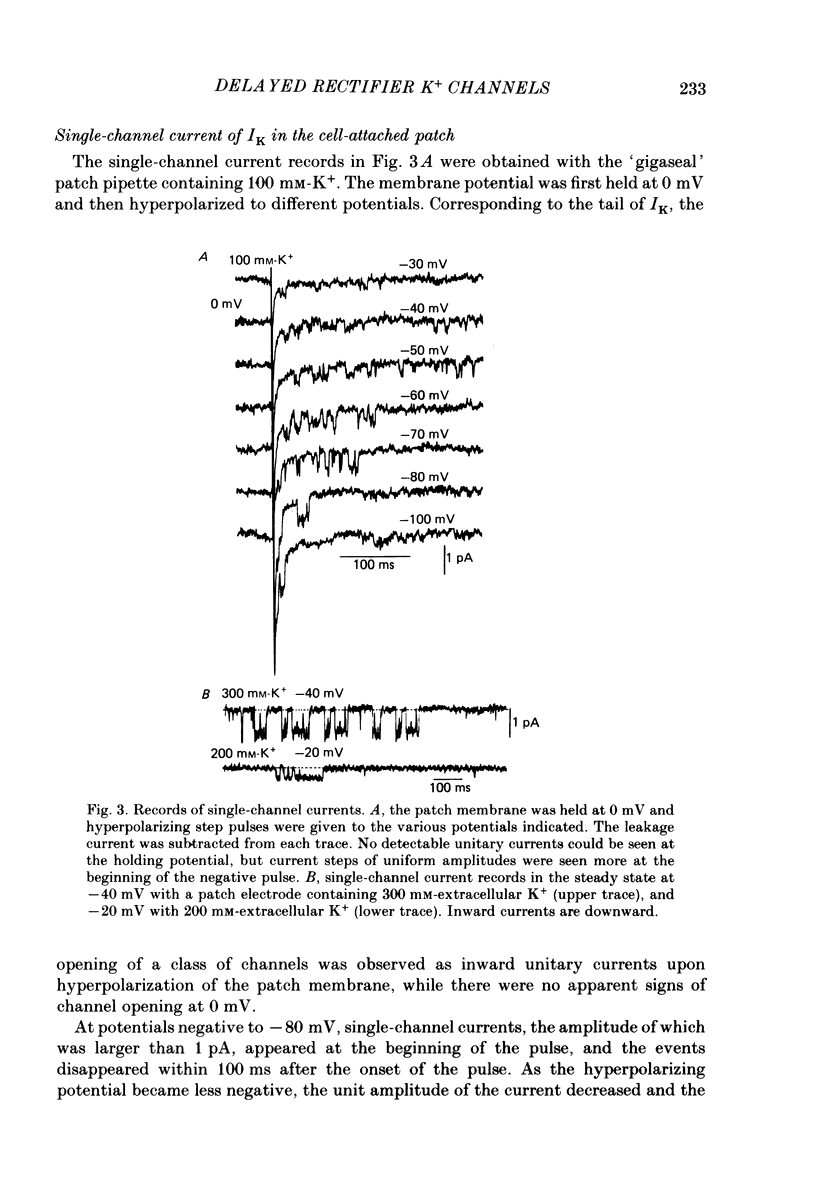
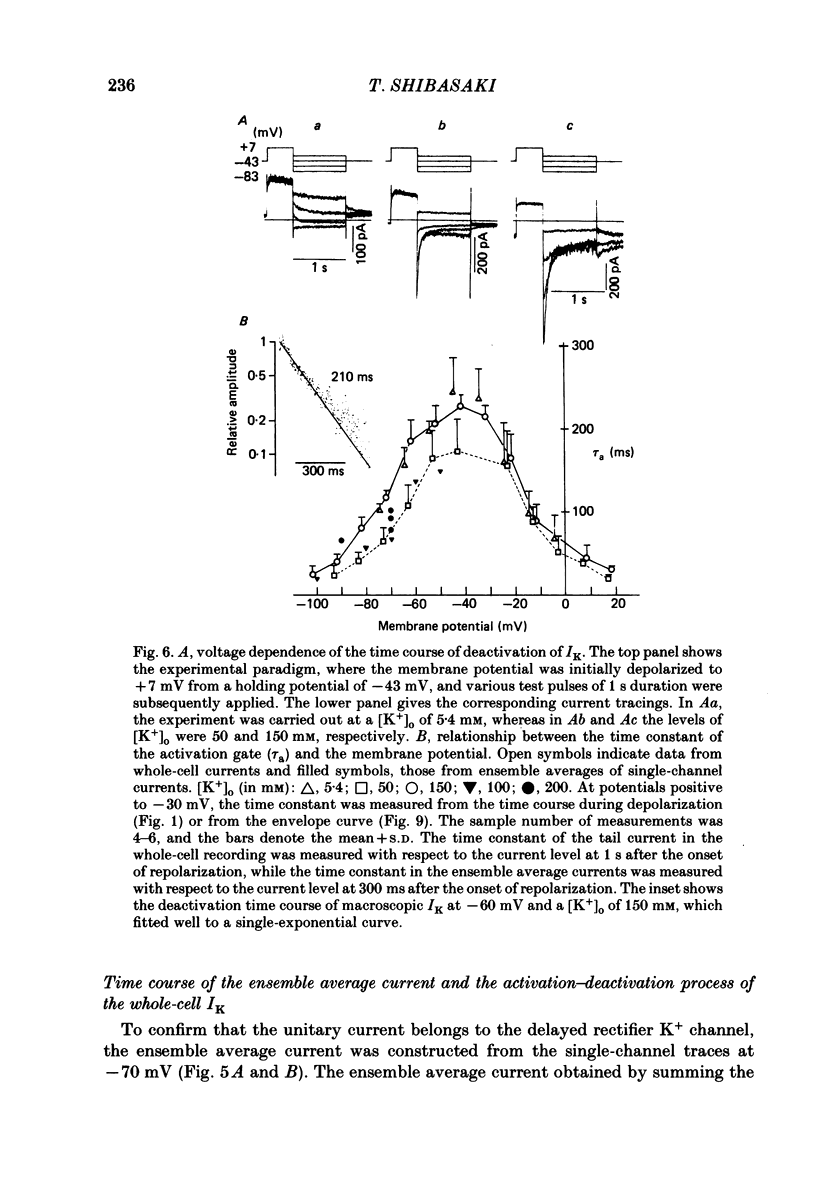
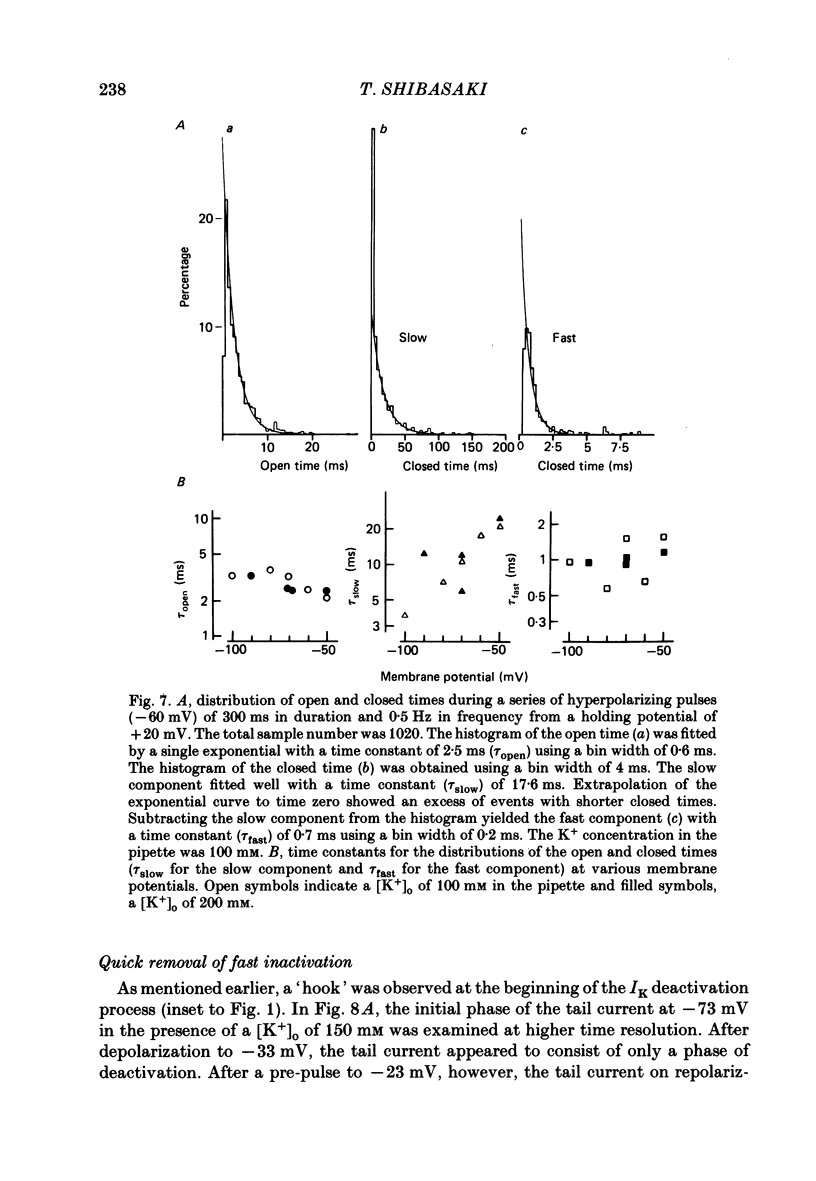
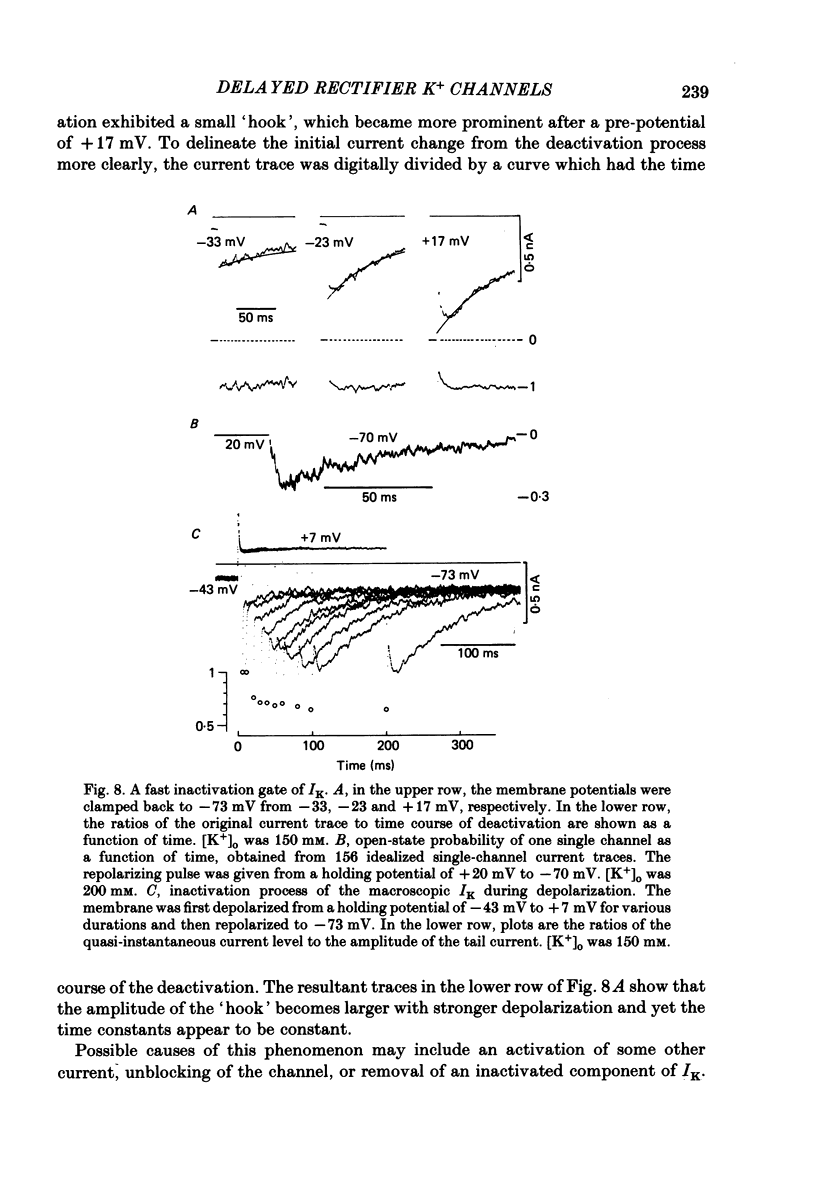
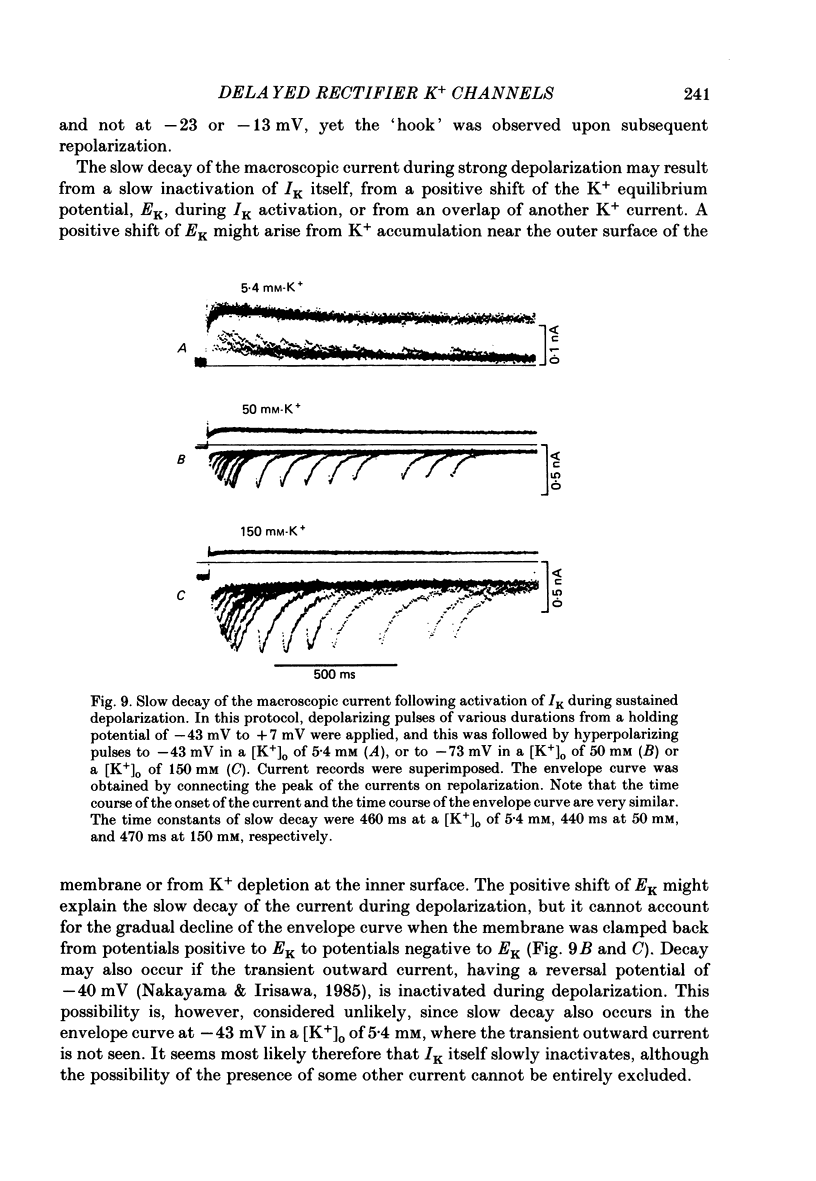
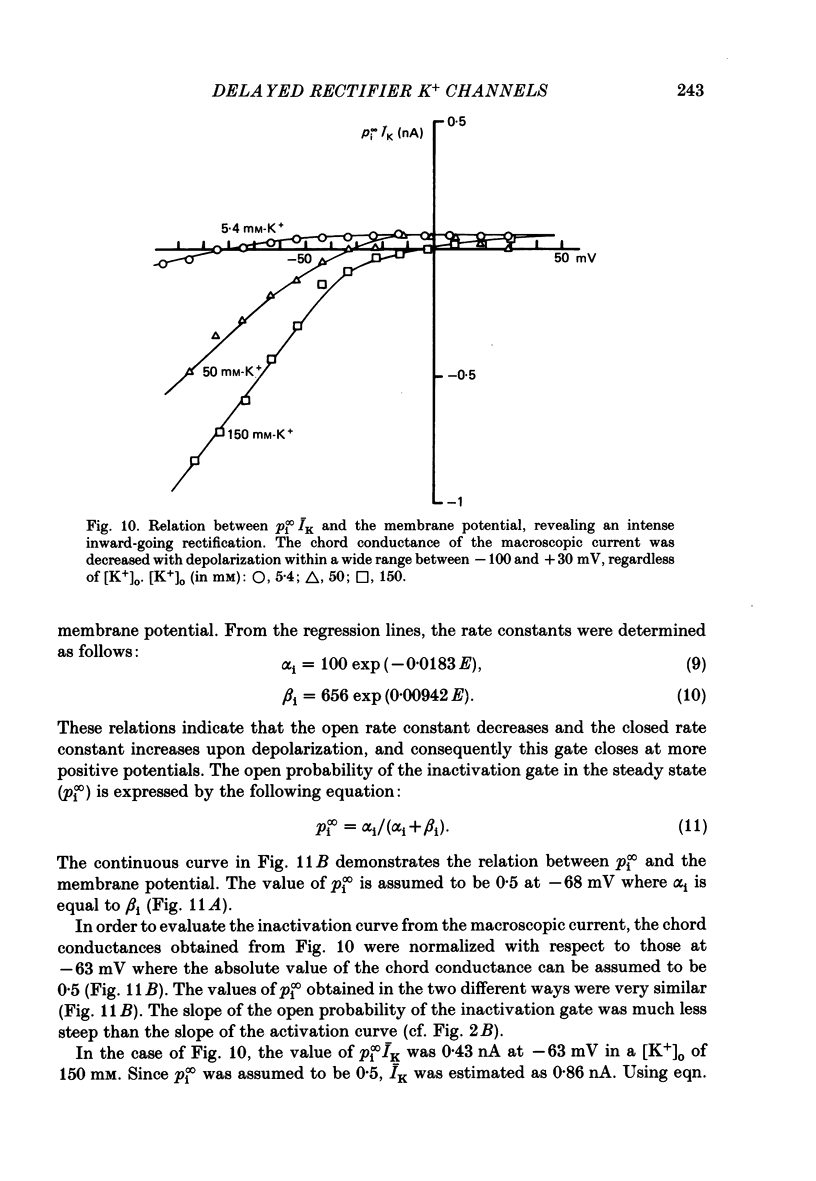
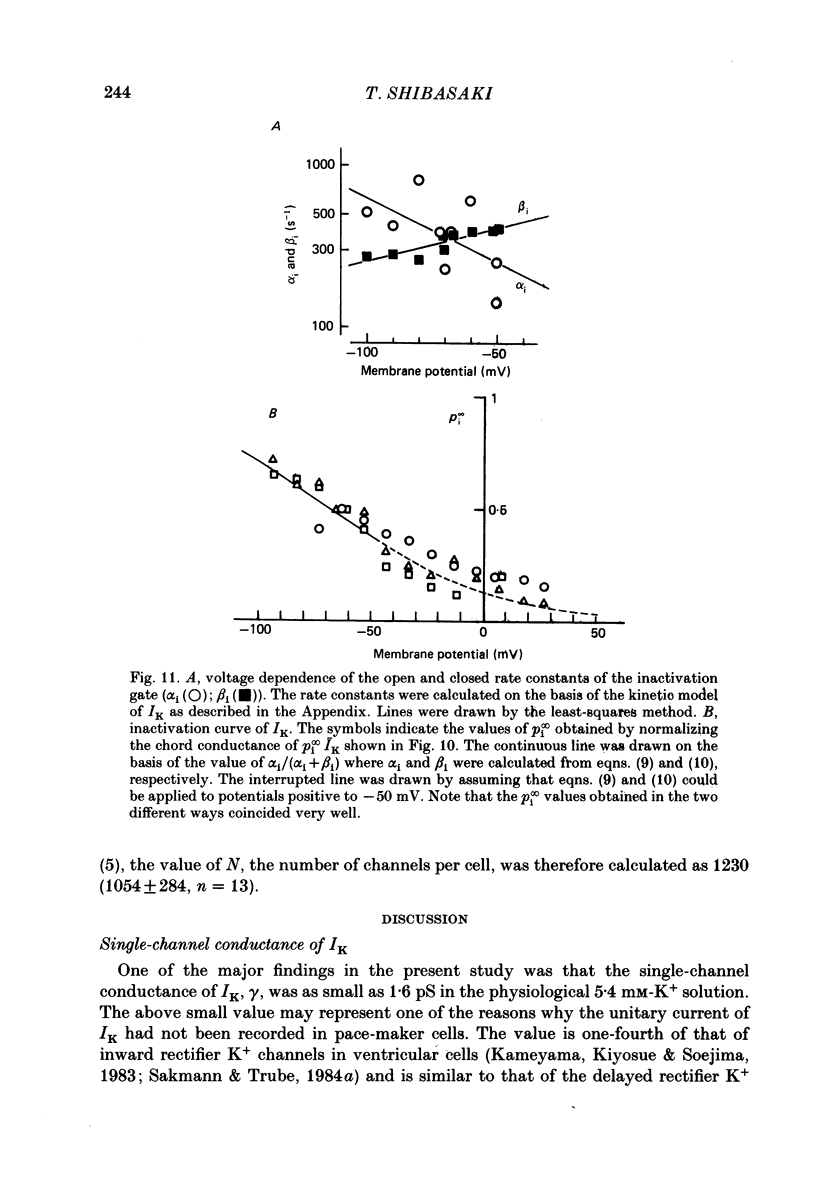
1. The delayed rectifier K+ current (IK) of single pace-maker cells from the sino-atrial node and the atrioventricular node of the rabbit heart was investigated using the whole-cell and cell-attached configurations of the patch-clamp technique. 2. The activation kinetics of the macroscopic IK were not altered by varying the extracellular K+ concentration ([K+]o) between 5.4 and 150 mM. The amplitude of the tail current of IK, however, was about 10-fold larger at a [K+]o of 150 mM than that at a [K+]o of 5.4 mM. 3. By using a high-[K+]o solution, inward single-channel currents were observed on repolarization from potentials positive to -40 mV. The current-voltage (I-V) relation was linear over the negative potential range and the reversal potential estimated by extrapolating the I-V curve was shifted by about 60 mV for a 10-fold increase in [K+]o, indicating that the channel was highly selective for K+. 4. The single-channel conductance was 11.1 pS at a [K+]o of 150 mM and varied in proportion to the square root of [K+]o. The total number of channels was estimated as approximately 1000 per cell (0.7/micron 2). On repolarization, the averaged single-channel current disappeared with a time constant similar to that of the macroscopic tail current of IK. 5. At potentials between -50 and -100 mV, the open and closed times of the single channel fitted well with single-exponential and biexponential distributions, respectively. As the membrane was progressively depolarized, the open time was shortened while the closed time was prolonged, suggesting a decrease of open probability. These changes were in the opposite direction to those expected from the delayed rectifier K+ current which progressively increases in magnitude at more positive potentials. 6. At the beginning of the macroscopic tail current, a transient increase of the inward current was found to precede the time-dependent decrease. This rapid initial change can be attributed to a quick removal of inactivation of IK which had occurred during the depolarizing pulse. This inactivation gate of the channel has very fast kinetics and could be responsible for the inward-going rectification observed in the 'fully activated' IK.
Full text
PDF


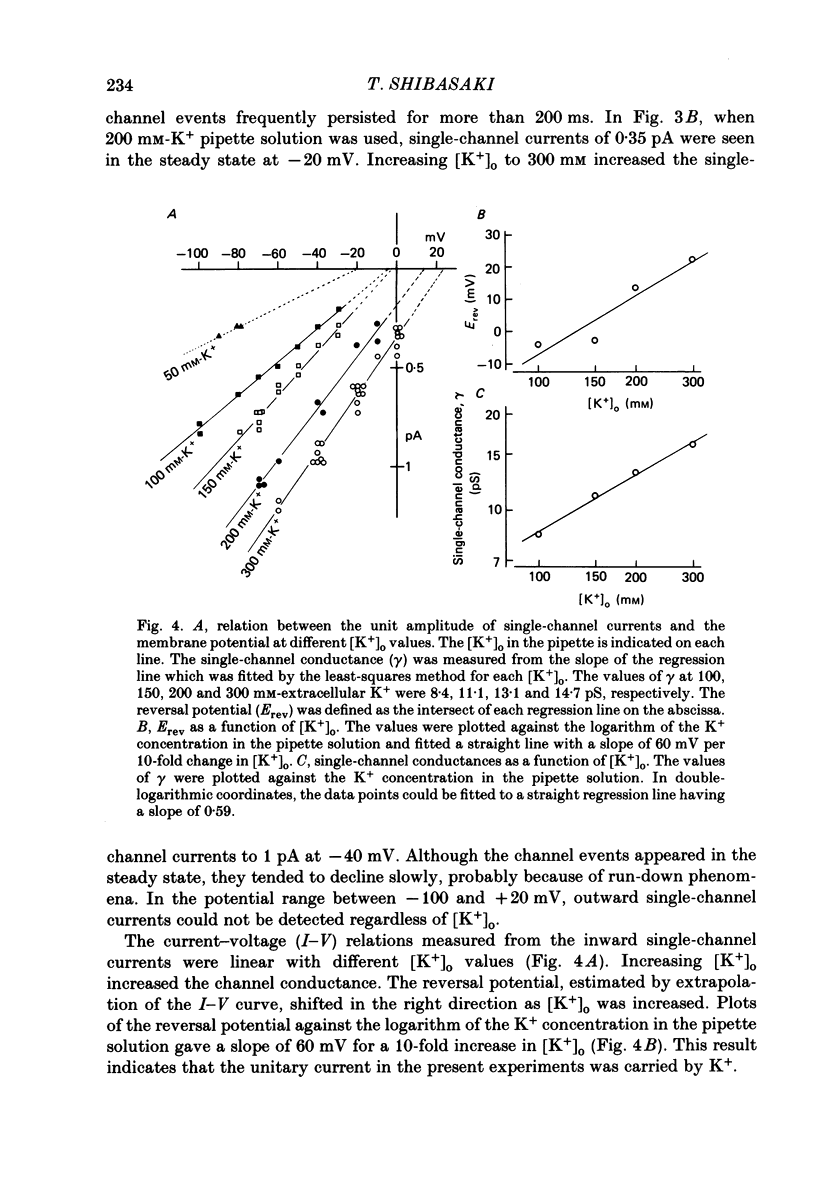
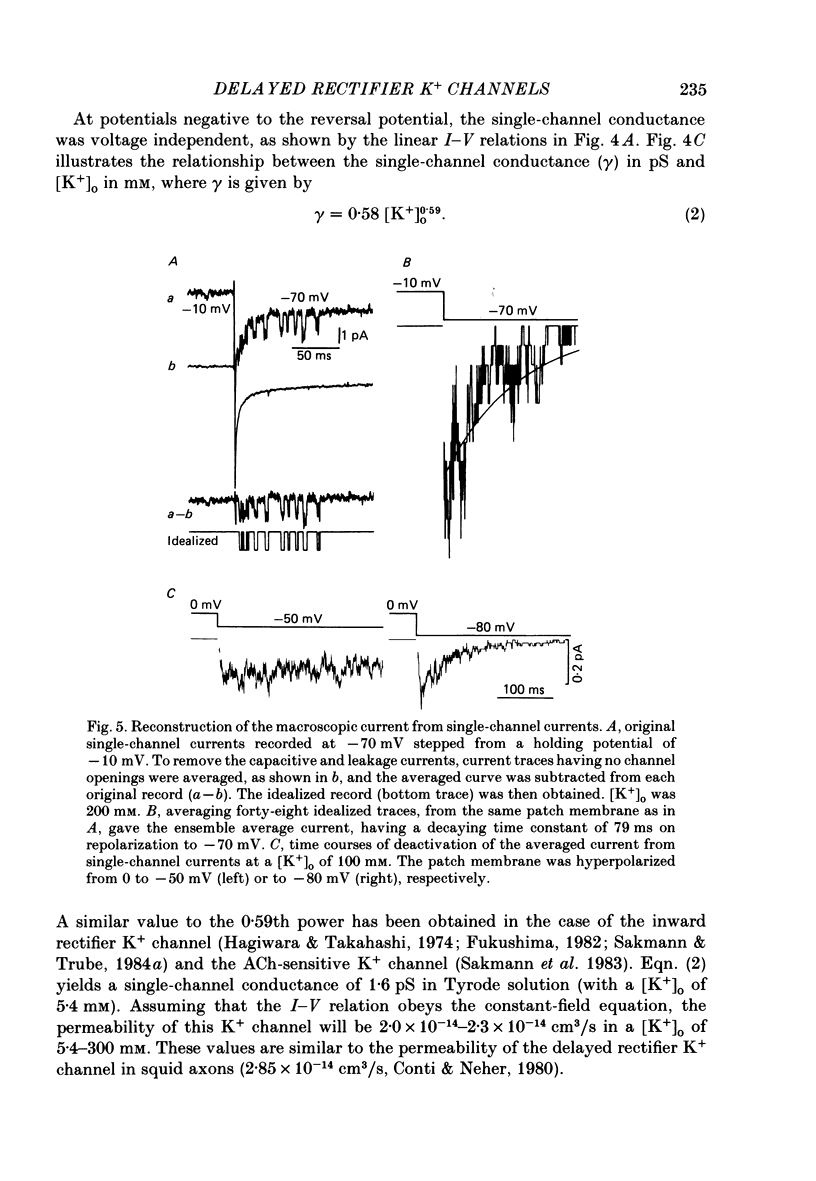




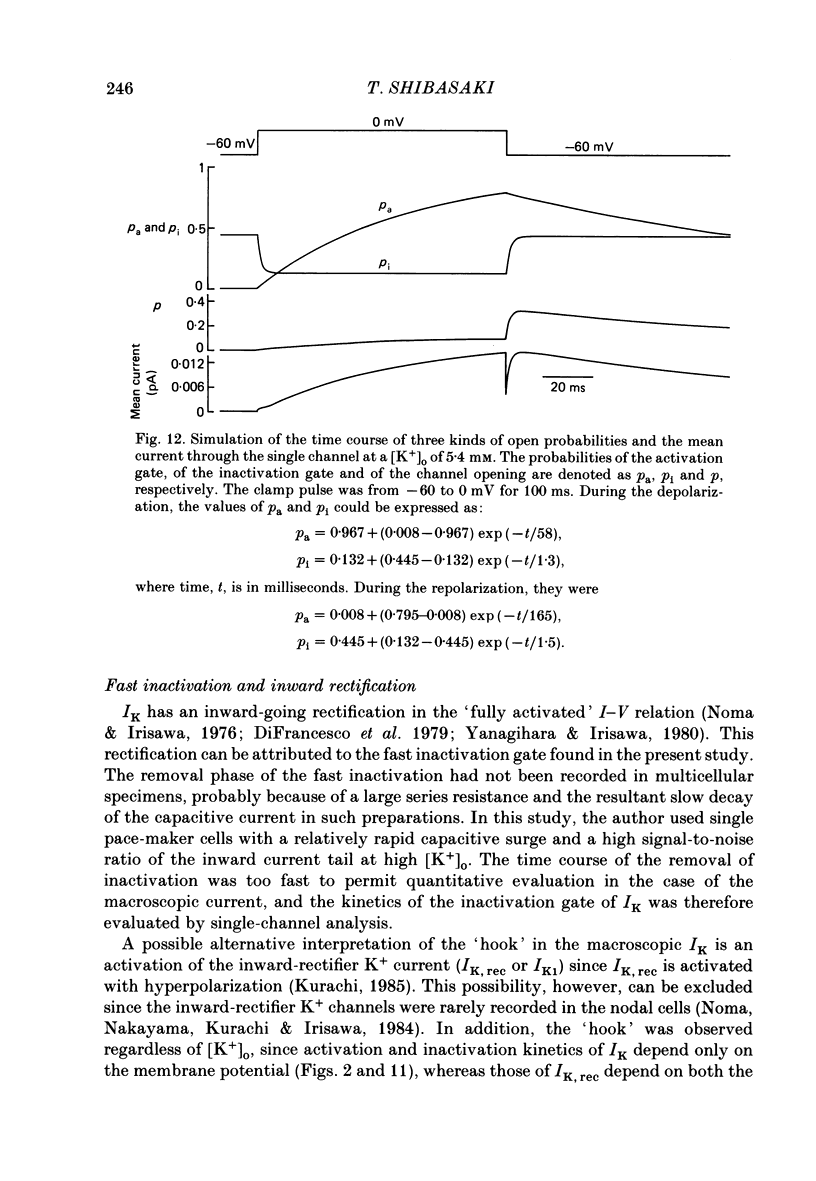



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Defelice L. J. Voltage-activated k channels in embryonic chick heart. Biophys J. 1984 Jan;45(1):40–42. doi: 10.1016/S0006-3495(84)84099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noma A., Trautwein W. Kinetics and magnitude of the time-dependent potassium current in the rabbit sinoatrial node: effect of external potassium. Pflugers Arch. 1979 Sep;381(3):271–279. doi: 10.1007/BF00583259. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. J Physiol. 1982 Oct;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978 Apr;58(2):461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984 Jul;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Nishimura M., Noma A., Irisawa H. Membrane currents in the rabbit atrioventricular node cell. Pflugers Arch. 1982 Mar;393(1):15–22. doi: 10.1007/BF00582385. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Hama K., Asai J., Irisawa H. Observations on the fine structure of nodal, Purkinje and working myocardial cells isolated from rabbit hearts. Arch Histol Jpn. 1986 Mar;49(1):105–116. doi: 10.1679/aohc.49.105. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Irisawa H. Transient outward current carried by potassium and sodium in quiescent atrioventricular node cells of rabbits. Circ Res. 1985 Jul;57(1):65–73. doi: 10.1161/01.res.57.1.65. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Kurachi Y., Noma A., Irisawa H. Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch. 1984 Nov;402(3):248–257. doi: 10.1007/BF00585507. [DOI] [PubMed] [Google Scholar]
- Noble D., Noble S. J. A model of sino-atrial node electrical activity based on a modification of the DiFrancesco-Noble (1984) equations. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):295–304. doi: 10.1098/rspb.1984.0065. [DOI] [PubMed] [Google Scholar]
- Noma A., Irisawa H. A time- and voltage-dependent potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1976 Nov 5;366(2-3):251–258. doi: 10.1007/BF00585886. [DOI] [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F. Ion conductance and ion selectivity of potassium channels in snail neurones. J Membr Biol. 1980 Dec 15;57(2):103–118. doi: 10.1007/BF01868997. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi J., Kokubun S., Noma A., Irisawa H. Spontaneously active cells isolated from the sino-atrial and atrio-ventricular nodes of the rabbit heart. Jpn J Physiol. 1981;31(4):547–558. doi: 10.2170/jjphysiol.31.547. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Potassium current during the pacemaker depolarization in rabbit sinoatrial node cell. Pflugers Arch. 1980 Dec;388(3):255–260. doi: 10.1007/BF00658491. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Noma A., Irisawa H. Reconstruction of sino-atrial node pacemaker potential based on the voltage clamp experiments. Jpn J Physiol. 1980;30(6):841–857. doi: 10.2170/jjphysiol.30.841. [DOI] [PubMed] [Google Scholar]


