Abstract
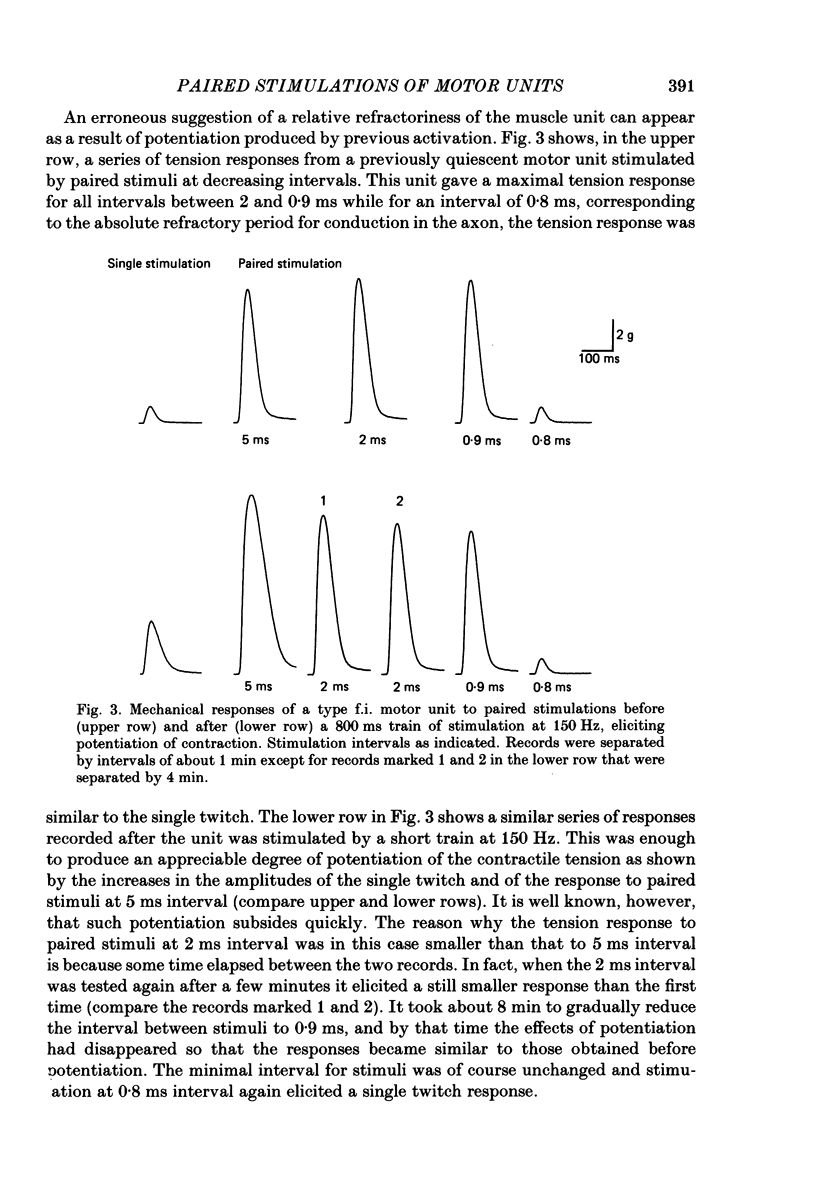
1. In adult cats, paired stimulations at short intervals were applied in ventral root filaments to single motor axons innervating the peroneus tertius muscle. Paired impulses were recorded from the muscle nerve simultaneously with the electrical and mechanical responses of the muscle portion of the motor unit (muscle unit). The interstimulus interval was gradually reduced in order to determine the minimum compatible with a full activation of the muscle unit by the second impulse. 2. For motor units of all physiological types, this minimum stimulus interval was the shortest interval allowing initiation and conduction of two impulses in the axon, that is, the absolute refractory period for conduction. Its duration ranged between 0.58 and 0.88 ms, displaying no correlation with the axonal conduction velocity. 3. The amount of tension enhancement produced by paired stimulations at the shortest interval varied with the type of the motor unit: it was largest for fast-fatigable units, intermediate for fast-resistant units and smallest for slow units. 4. Paired impulses elicited by paired stimulations at the shortest possible interval arrived near the muscle at a longer interval because the second impulse was conducted at a slower velocity. The minimum interval between arrival of impulses at the muscle depended on conduction velocity and on conduction distance. 5. In motor axons to peroneus tertius, paired impulses leaving the spinal cord at a mean interval of 0.78 ms arrived near the muscle separated by a mean interval of 1.90 ms. Since such an interval always allowed full activation of the muscle unit by the second impulse, this interval is longer than the refractory period of motor units in this muscle.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. The recovery process of excitable tissues: Part II. J Physiol. 1921 Aug 3;55(3-4):193–225. doi: 10.1113/jphysiol.1921.sp001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. The effect on a muscle twitch of the back-response of its motor nerve fibres. J Physiol. 1960 Feb;150:332–346. doi: 10.1113/jphysiol.1960.sp006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. Axonal refractory period of single short toe extensor motor units in man. J Neurol Neurosurg Psychiatry. 1980 Oct;43(10):917–924. doi: 10.1136/jnnp.43.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. Effects of prior activity on the conduction in single motor units in man. J Neurol Neurosurg Psychiatry. 1983 Apr;46(4):317–321. doi: 10.1136/jnnp.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. Refractory period of single motor nerve fibres in man. J Neurol Neurosurg Psychiatry. 1984 Apr;47(4):344–348. doi: 10.1136/jnnp.47.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller A. J., Proske U. Further observations on back-firing in the motor nerve fibres of a muscle during twitch contractions. J Physiol. 1978 Dec;285:59–69. doi: 10.1113/jphysiol.1978.sp012557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Rudomin P., Zajac F. E., 3rd Catch property in single mammalian motor units. Science. 1970 Apr 3;168(3927):122–124. doi: 10.1126/science.168.3927.122. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Rudomin P., Zajac F. E., 3rd The effect of activation history on tension production by individual muscle units. Brain Res. 1976 Jun 18;109(3):515–529. doi: 10.1016/0006-8993(76)90031-7. [DOI] [PubMed] [Google Scholar]
- Cooper S. The isometric responses of mammalian muscles. J Physiol. 1930 Jun 27;69(4):377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., O'connor W. J. Responses which nerve impulses evoke in mammalian striated muscles. J Physiol. 1939 Nov 14;97(1):44–102. doi: 10.1113/jphysiol.1939.sp003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A. F., Luschei E. S. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol. 1970 May;33(3):382–392. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- Gogan P., Gustafsson B., Jankowska E., Tyc-Dumont S. On re-excitation of feline motoneurones: its mechanism and consequences. J Physiol. 1984 May;350:81–91. doi: 10.1113/jphysiol.1984.sp015189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F. The delay of the electrical response of nerve to a second stimulus. J Physiol. 1910 May 11;40(3):250–274. doi: 10.1113/jphysiol.1910.sp001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G. Rabbit diaphragm: two types of fibres determined by calcium strontium activation and protein content. J Physiol. 1979 Oct;295:345–352. doi: 10.1113/jphysiol.1979.sp012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J., Zytnicki D. After-effects of repetitive stimulation at low frequency on fast-contracting motor units of cat muscle. J Physiol. 1983 Jul;340:129–143. doi: 10.1113/jphysiol.1983.sp014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J., Zytnicki D. Distribution of physiological types of motor units in the cat peroneus tertius muscle. Exp Brain Res. 1982;48(2):177–184. doi: 10.1007/BF00237213. [DOI] [PubMed] [Google Scholar]
- Kimura J. A method for estimating the refractory period of motor fibers in the human peripheral nerve. J Neurol Sci. 1976 Aug;28(4):485–490. doi: 10.1016/0022-510x(76)90119-2. [DOI] [PubMed] [Google Scholar]
- Kopec J., Delbeke J., McComas A. J. Refractory period studies in a human neuromuscular preparation. J Neurol Neurosurg Psychiatry. 1978 Jan;41(1):54–64. doi: 10.1136/jnnp.41.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. M. The effect of denervation on the mechanical and electrical responses of fast and slow mammalian twitch muscle. J Physiol. 1972 Apr;222(1):51–75. doi: 10.1113/jphysiol.1972.sp009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K. On the refractory period of muscle and nerve. J Physiol. 1909 Dec 23;39(5):331–340. doi: 10.1113/jphysiol.1909.sp001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath G. J., Matthews P. B. Evidence from the use of vibration during procaine nerve block that the spindle group II fibres contribute excitation to the tonic stretch reflex of the decerebrate cat. J Physiol. 1973 Dec;235(2):371–408. doi: 10.1113/jphysiol.1973.sp010392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan M. J., Hoffer J. A., Loeb G. E. Physiological characterization of motor unit properties in intact cats. J Neurosci Methods. 1983 Feb;7(2):137–149. doi: 10.1016/0165-0270(83)90076-6. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. The influence of diameter of medullated nerve fibres of cats on the rising and falling phases of the spike and its recovery. J Physiol. 1966 Jun;184(4):791–811. doi: 10.1113/jphysiol.1966.sp007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W., Richter A., Grippo J. Responses of neurones in cat's abducens nuclei to horizontal angular acceleration. Pflugers Arch. 1969 Jun 19;309(4):285–309. doi: 10.1007/BF00587754. [DOI] [PubMed] [Google Scholar]


