Abstract
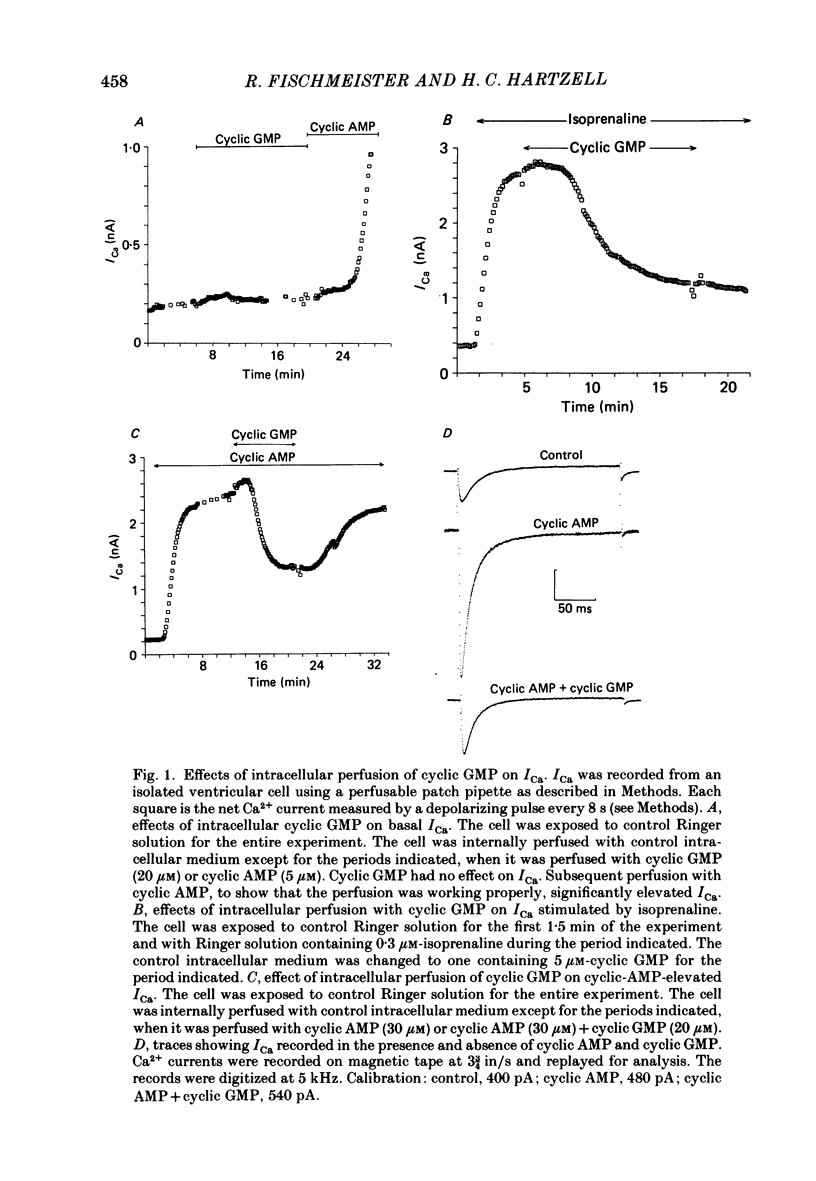
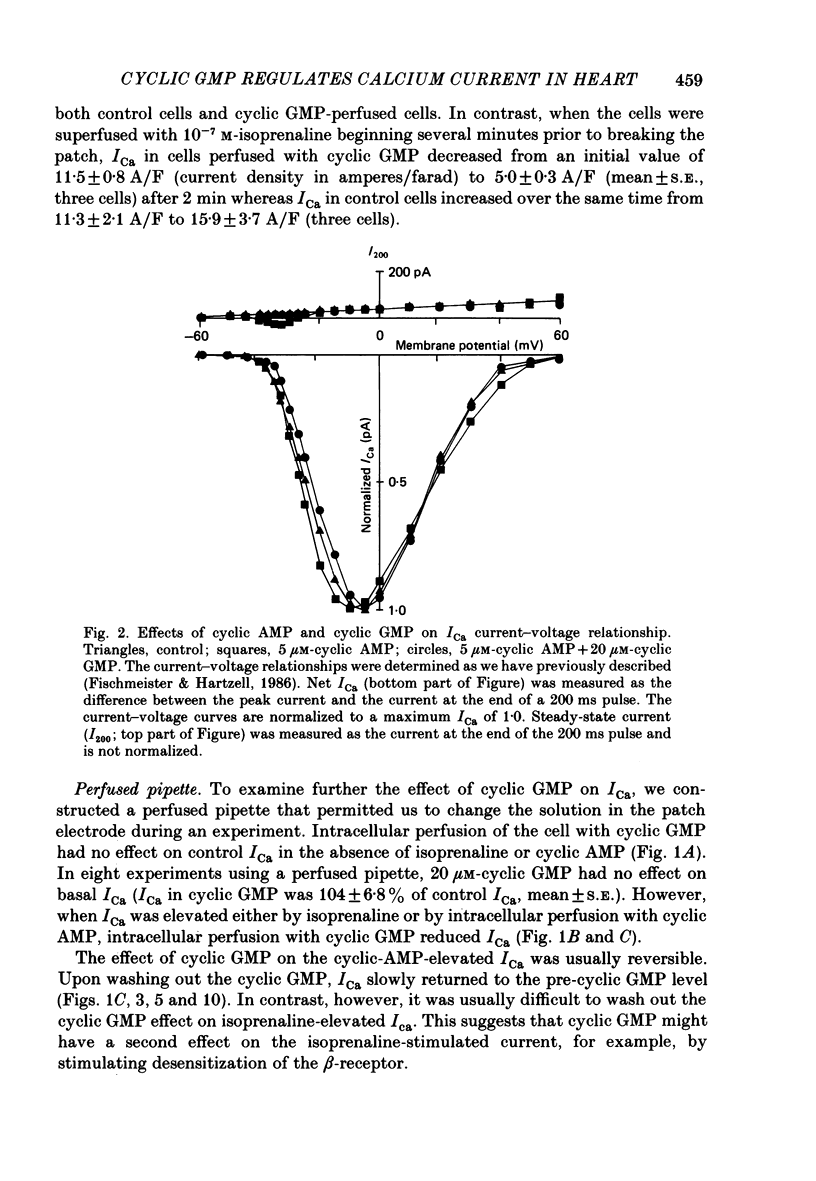
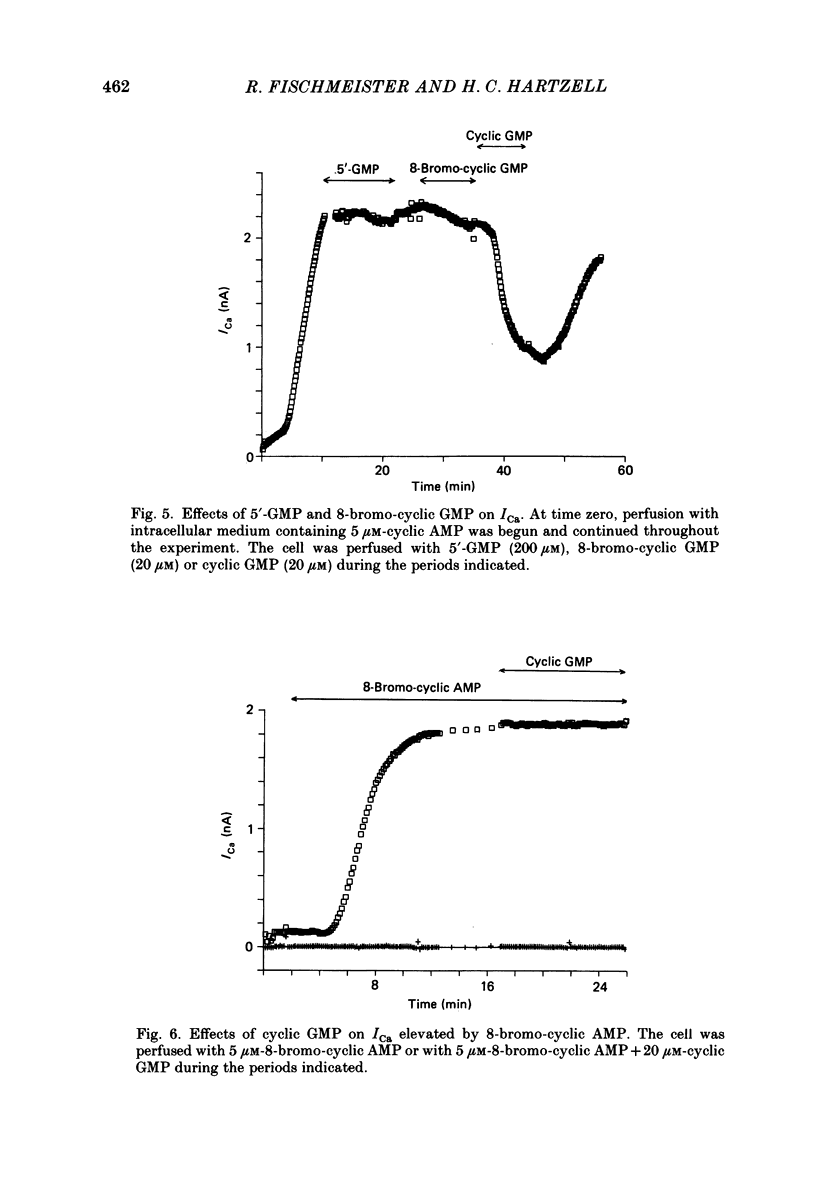
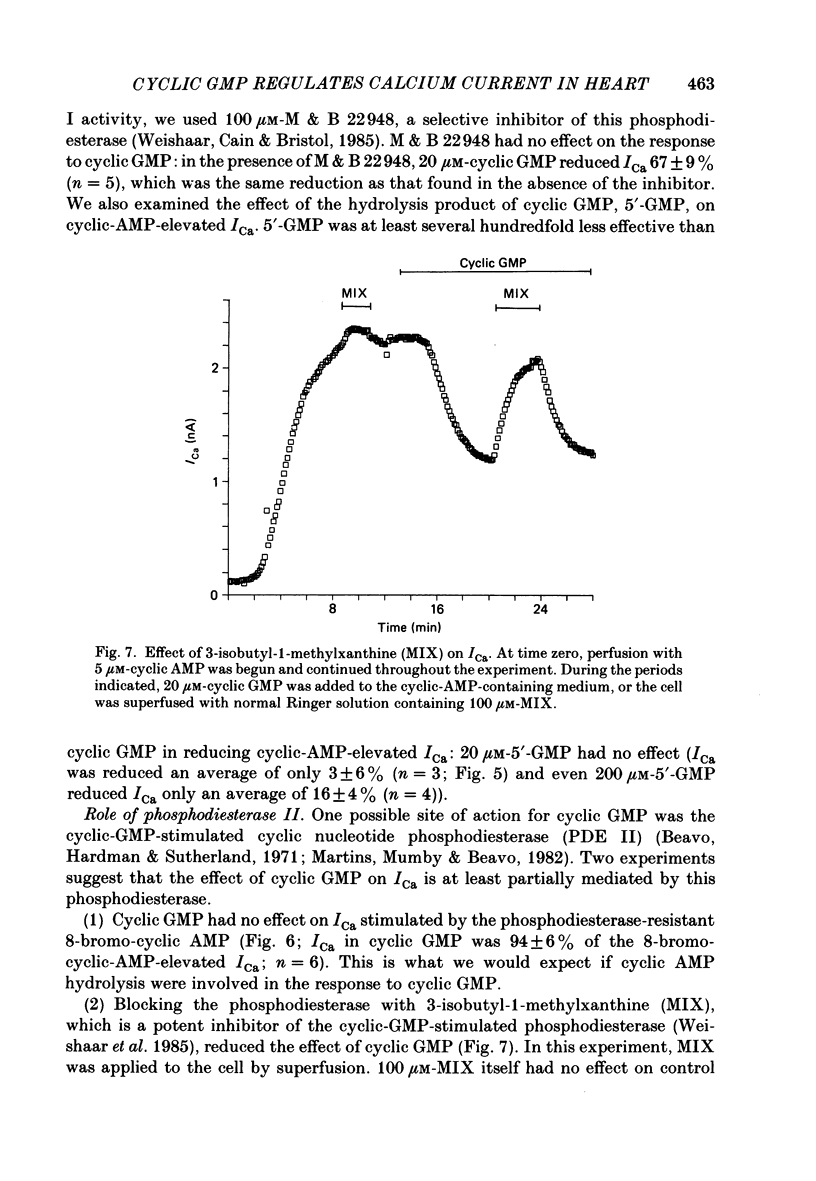
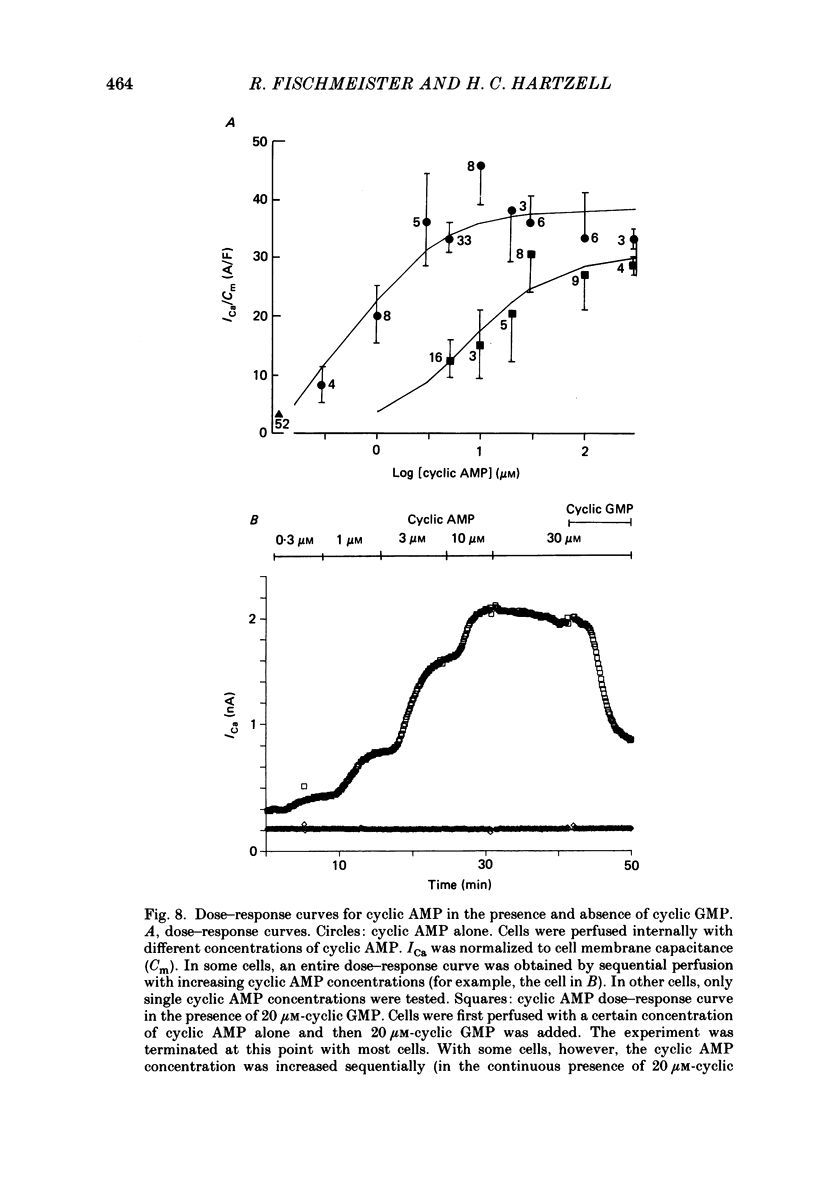
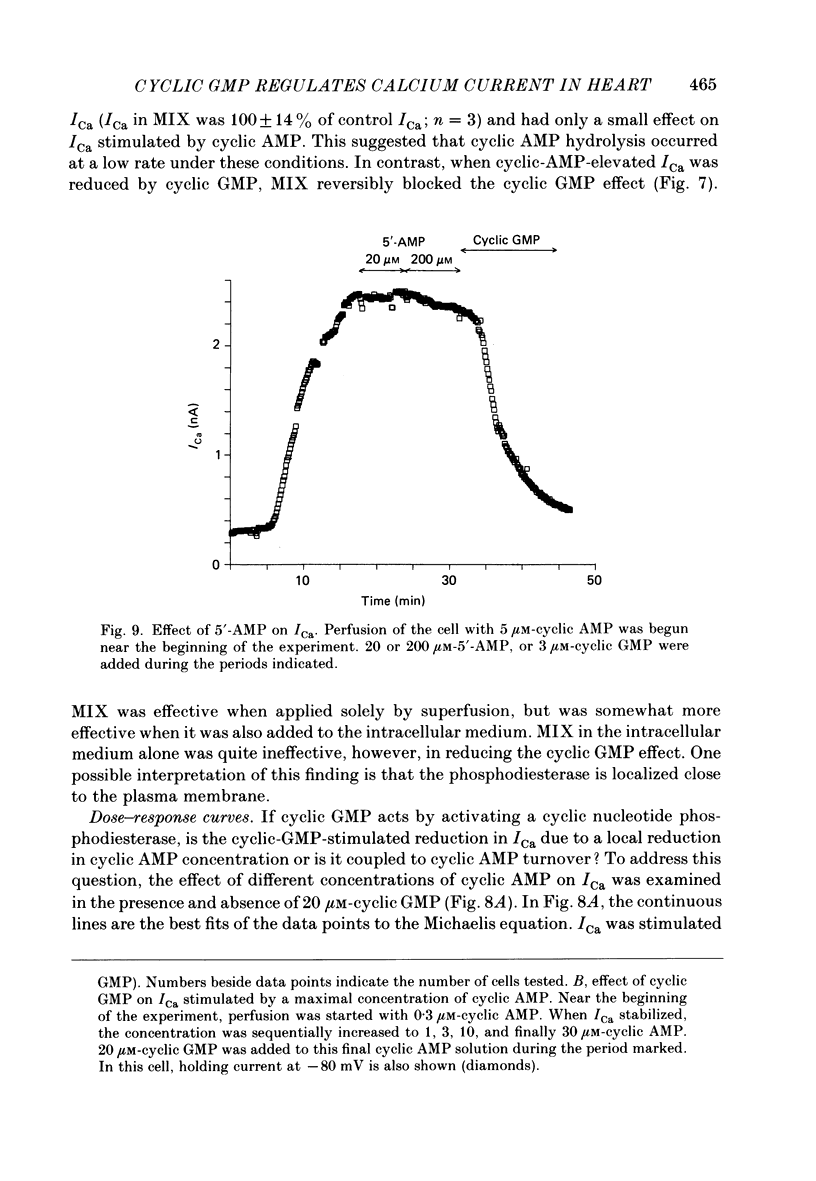
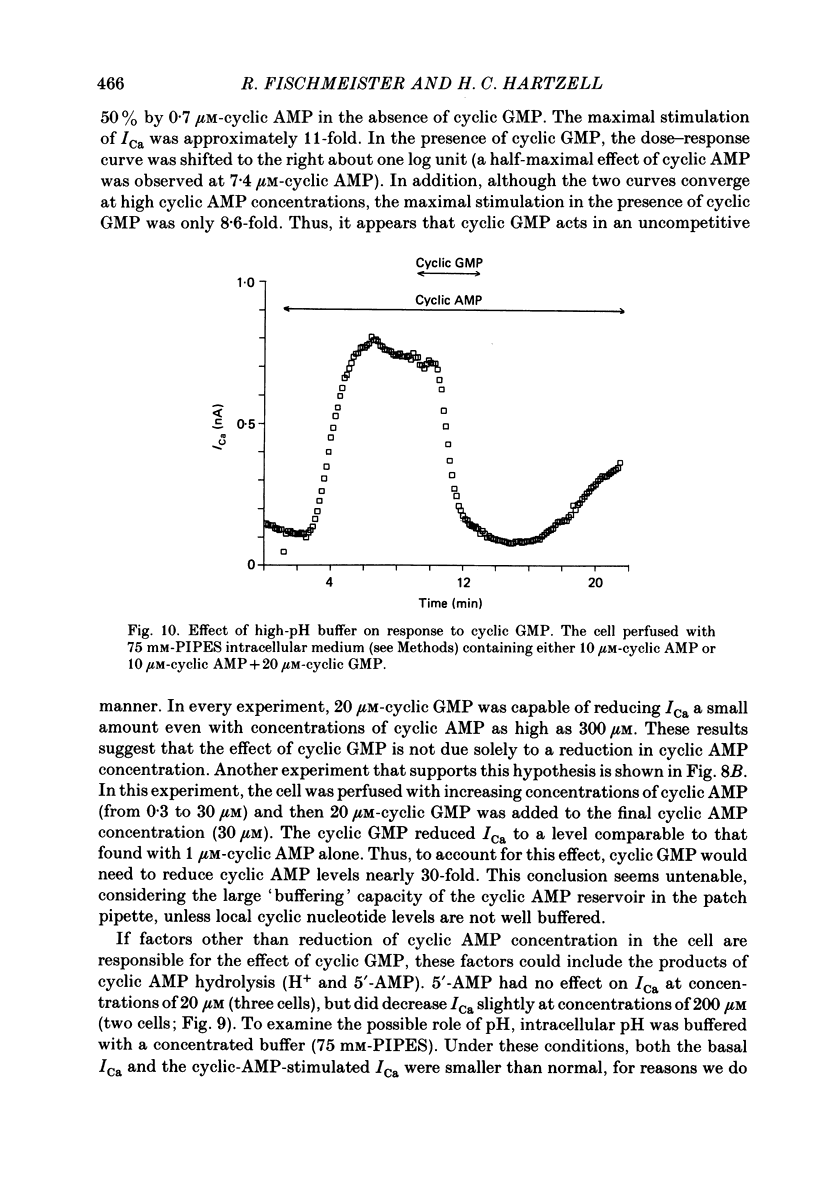
1. The effect of intracellular perfusion with cyclic AMP and cyclic GMP on Ca2+ current (ICa) was studied in single cells isolated from frog ventricle using the whole-cell patch-clamp technique and a perfused pipette. 2. Intracellular perfusion with cyclic GMP (0.1-20 microM) had no effect on the basal ICa. However, when ICa was increased by isoprenaline or by intracellular perfusion with cyclic AMP, perfusion with cyclic GMP (20 microM) reduced ICa by an average of 67%. The effect of cyclic GMP on ICa elevated by cyclic AMP was reversible. A half-maximal effect of cyclic GMP was observed at 0.6 microM. Cyclic GMP had no significant effect on the shape of the ICa current-voltage relationship. 3. The effect of cyclic GMP was specific to the 3',5' form; 2',3'-cyclic GMP had no effect. 4. The effect of cyclic GMP was apparently not mediated by stimulation of cyclic-GMP-dependent protein kinase because 8-bromo-cyclic GMP, a very potent activator of the protein kinase, was without effect. 5. Cyclic GMP had no effect on ICa elevated by the non-hydrolysable 8-bromo-cyclic AMP. The effect of cyclic GMP on cyclic-AMP-elevated ICa was partially blocked by the phosphodiesterase inhibitor, methylisobutylxanthine. Thus, it was hypothesized that the effect of cyclic GMP was mediated by hydrolysis of cyclic AMP as a result of a stimulation of a cyclic nucleotide phosphodiesterase by cyclic GMP. 6. The dose-response curve for cyclic AMP on ICa was well fitted by the Michaelis equation with a K50 (i.e. concentration of cyclic AMP at which response is 50% of the maximum) of 0.7 microM and a maximal 11-fold stimulation of ICa. Cyclic GMP shifted the curve one log unit to the right and decreased the maximal stimulation to 8.6-fold. Thus, the effect of cyclic GMP appeared uncompetitive. 7. The products of cyclic AMP and cyclic GMP hydrolysis, 5'-AMP and 5'-GMP, had no effect on ICa. Furthermore, strong buffering of intracellular pH did not reduce the effect of cyclic GMP. 8. It is proposed that cyclic-GMP-stimulation of a cyclic nucleotide phosphodiesterase may be one of several mechanisms by which acetylcholine regulates ICa.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Stimulation of adenosine 3',5'-monophosphate hydrolysis by guanosine 3',5'-monophosphate. J Biol Chem. 1971 Jun 25;246(12):3841–3846. [PubMed] [Google Scholar]
- Bkaily G., Sperelakis N. Injection of guanosine 5'-cyclic monophosphate into heart cells blocks calcium slow channels. Am J Physiol. 1985 May;248(5 Pt 2):H745–H749. doi: 10.1152/ajpheart.1985.248.5.H745. [DOI] [PubMed] [Google Scholar]
- Brooker G. Dissociation of cyclic GMP from the negative inotropic action of carbachol in guinea pig atria. J Cyclic Nucleotide Res. 1977 Dec;3(6):407–413. [PubMed] [Google Scholar]
- Brown B. S., Polson J. B., Krzanowski J. J., Wiggins J. R. Influence of isoproterenol and methylisobutylxanthine on the contractile and cyclic nucleotide effects of methacholine in isolated rat atria. J Pharmacol Exp Ther. 1980 Feb;212(2):325–332. [PubMed] [Google Scholar]
- Corbin J. D., Ogreid D., Miller J. P., Suva R. H., Jastorff B., Døskeland S. O. Studies of cGMP analog specificity and function of the two intrasubunit binding sites of cGMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):1208–1214. [PubMed] [Google Scholar]
- Diamond J., Chu E. B. A novel cyclic GMP-lowering agent, LY83583, blocks carbachol-induced cyclic GMP elevation in rabbit atrial strips without blocking the negative inotropic effects of carbachol. Can J Physiol Pharmacol. 1985 Aug;63(8):908–911. doi: 10.1139/y85-150. [DOI] [PubMed] [Google Scholar]
- Diamond J., Ten Eick R. E., Trapani A. J. Are increases in cyclic GMP levels responsible for the negative inotropic effects of acetylcholine in the heart? Biochem Biophys Res Commun. 1977 Dec 7;79(3):912–918. doi: 10.1016/0006-291x(77)91197-4. [DOI] [PubMed] [Google Scholar]
- Dobson J. G., Jr The effect of acetylcholine, ischemia, and anoxia on rat heart purine cyclic nucleotides and contractility. Circ Res. 1981 Oct;49(4):912–922. doi: 10.1161/01.res.49.4.912. [DOI] [PubMed] [Google Scholar]
- Endoh M., Maruyama M., Iijima T. Attenuation of muscarinic cholinergic inhibition by islet-activating protein in the heart. Am J Physiol. 1985 Aug;249(2 Pt 2):H309–H320. doi: 10.1152/ajpheart.1985.249.2.H309. [DOI] [PubMed] [Google Scholar]
- Endoh M., Shimizu T. Failure of dibutyryl and 8-bromo-cyclic GMP to mimic the antagonistic action of carbachol on the positive inotropic effects of sympathomimetic amines in the canine isolated ventricular myocardium. Jpn J Pharmacol. 1979 Jun;29(3):423–433. doi: 10.1254/jjp.29.423. [DOI] [PubMed] [Google Scholar]
- Endoh M., Yamashita S. Differential responses to carbachol, sodium nitroprusside and 8-bromo-guanosine 3',5'-monophosphate of canine atrial and ventricular muscle. Br J Pharmacol. 1981 Jun;73(2):393–399. doi: 10.1111/j.1476-5381.1981.tb10434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Couchie D., Dumont J. E., Baraniak J., Stec W. J., Abbad E. G., Petridis G., Jastorff B. Specificity of cyclic GMP activation of a multi-substrate cyclic nucleotide phosphodiesterase from rat liver. Eur J Biochem. 1981 Apr;115(3):503–510. doi: 10.1111/j.1432-1033.1981.tb06231.x. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming B. P., Giles W., Lederer J. Are acetylcholine-induced increases in 42K efflux mediated by intracellular cyclic GMP in turtle cardiac pace-maker tissue? J Physiol. 1981 May;314:47–64. doi: 10.1113/jphysiol.1981.sp013689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Evidence that cyclic GMP may regulate cyclic AMP metabolism in the isolated frog ventricle. J Mol Cell Cardiol. 1981 Nov;13(11):963–979. doi: 10.1016/0022-2828(81)90472-7. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Lincoln T. M., Corbin J. D. Characterization of a novel cGMP binding protein from rat lung. J Biol Chem. 1980 Jan 25;255(2):620–626. [PubMed] [Google Scholar]
- Gardner R. M., Allen D. O. Effect of acetylcholine on glycogen phosphorylase activity and cyclic nucleotide content in isolated perfused rat hearts. J Cyclic Nucleotide Res. 1976;2(3):171–178. [PubMed] [Google Scholar]
- George W. J., Ignarro L. J., Paddock R. J., White L., Kadowitz P. J. Oppositional effects of acetylcholine and isoproterenol on isometric tension and cyclic nucleotide concentrations in rabbit atria. J Cyclic Nucleotide Res. 1975;1(5):339–347. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari H., McCarl R. L. Involvement of cyclic nucleotides in the beating response of rat heart cells in culture. J Mol Cell Cardiol. 1976 Jun;8(6):481–488. doi: 10.1016/0022-2828(76)90021-3. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Ames A. A., 3rd, Gander J. E., Walseth T. F. Magnitude of increase in retinal cGMP metabolic flux determined by 18O incorporation into nucleotide alpha-phosphoryls corresponds with intensity of photic stimulation. J Biol Chem. 1983 Aug 10;258(15):9213–9219. [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Kohlhardt M., Haap K. 8-bromo-guanosine-3',5' -monophosphate mimics the effect of acetylcholine on slow response action potential and contractile force in mammalian atrial myocardium. J Mol Cell Cardiol. 1978 Jun;10(6):573–586. doi: 10.1016/0022-2828(78)90015-9. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- Lincoln T. M., Keely S. L. Regulation of cardiac cyclic GMP-dependent protein kinase. Biochim Biophys Acta. 1981 Aug 17;676(2):230–244. doi: 10.1016/0304-4165(81)90192-6. [DOI] [PubMed] [Google Scholar]
- Linden J., Brooker G. The questionable role of cyclic guanosine 3':5'-monophosphate in heart. Biochem Pharmacol. 1979 Dec 1;28(23):3351–3360. doi: 10.1016/0006-2952(79)90072-8. [DOI] [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- Martins T. J., Mumby M. C., Beavo J. A. Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J Biol Chem. 1982 Feb 25;257(4):1973–1979. [PubMed] [Google Scholar]
- Nargeot J., Nerbonne J. M., Engels J., Lester H. A. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath H. Does cyclic GMP mediate the negative inotropic effect of acetylcholine in the heart? Nature. 1977 May 5;267(5606):72–74. doi: 10.1038/267072a0. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Singh J., Flitney F. W. Inotropic responses of the frog ventricle to dibutyryl cyclic AMP and 8-bromo cyclic GMP and related changes in endogenous cyclic nucleotide levels. Biochem Pharmacol. 1981 Jun 15;30(12):1475–1481. doi: 10.1016/0006-2952(81)90370-1. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Cavalié A. Cardiac calcium channels and their control by neurotransmitters and drugs. J Am Coll Cardiol. 1985 Dec;6(6):1409–1416. doi: 10.1016/s0735-1097(85)80233-3. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Trube G. Negative inotropic effect of cyclic GMP in cardiac fiber fragments. Pflugers Arch. 1976 Nov 5;366(2-3):293–295. doi: 10.1007/BF00585895. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Wahler G. M., Sperelakis N. Intracellular injection of cyclic GMP depresses cardiac slow action potentials. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(1):83–95. [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Burrows S. D., Kobylarz D. C., Quade M. M., Evans D. B. Multiple molecular forms of cyclic nucleotide phosphodiesterase in cardiac and smooth muscle and in platelets. Isolation, characterization, and effects of various reference phosphodiesterase inhibitors and cardiotonic agents. Biochem Pharmacol. 1986 Mar 1;35(5):787–800. doi: 10.1016/0006-2952(86)90247-9. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- Wilkerson R. D., Paddock R. J., George W. J. Effects of derivatives of cyclic amp and cyclic gmp on contraction force of cat papillary muscles. Eur J Pharmacol. 1976 Mar;36(1):247–251. doi: 10.1016/0014-2999(76)90280-6. [DOI] [PubMed] [Google Scholar]
- Wrenn R. W., Kuo J. F. Cyclic GMP-dependent phosphorylation of an endogenous protein from rat heart. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1274–1280. doi: 10.1016/0006-291x(81)91585-0. [DOI] [PubMed] [Google Scholar]


