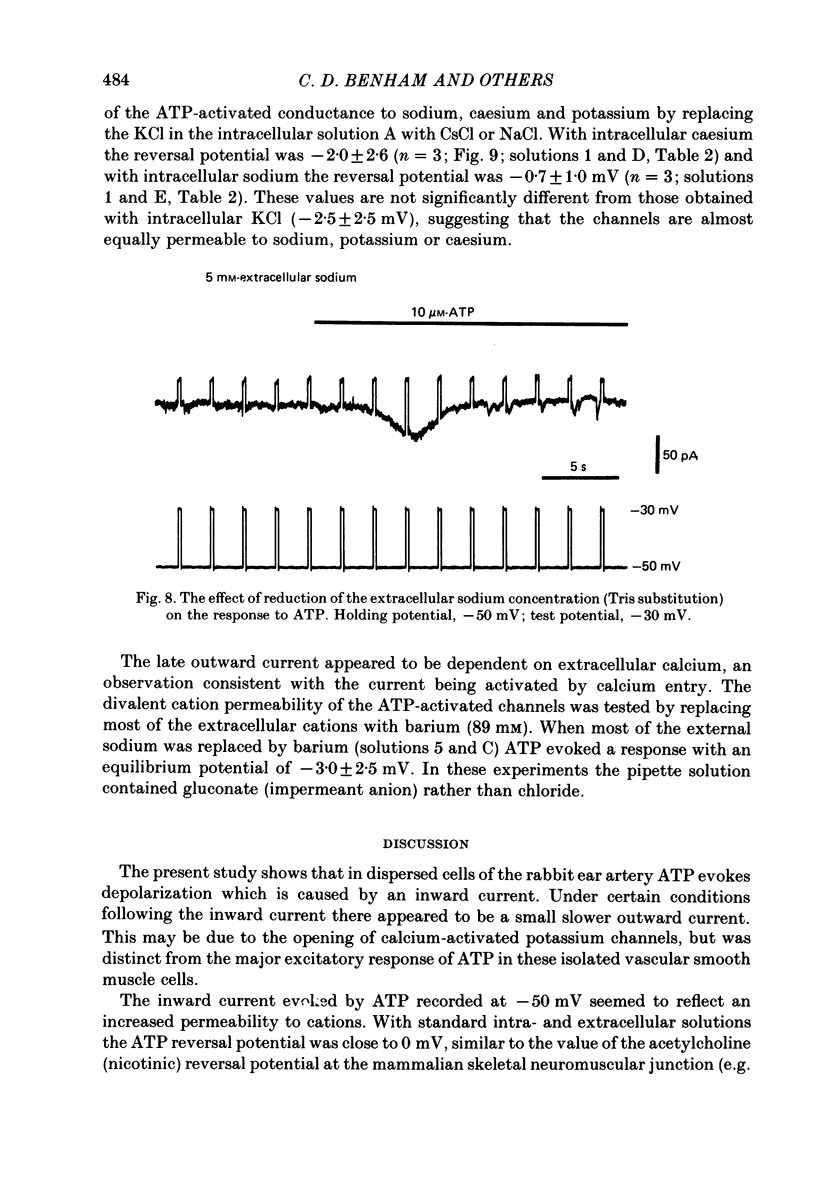
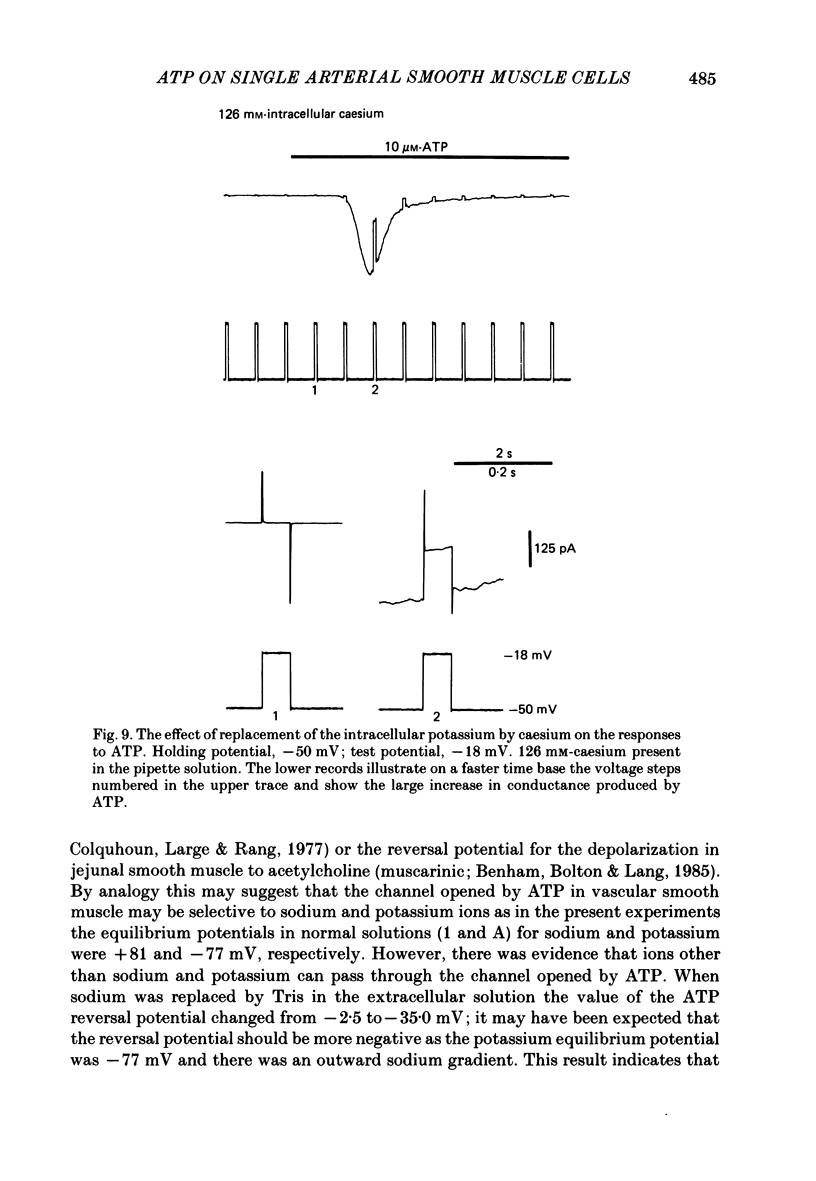
Abstract
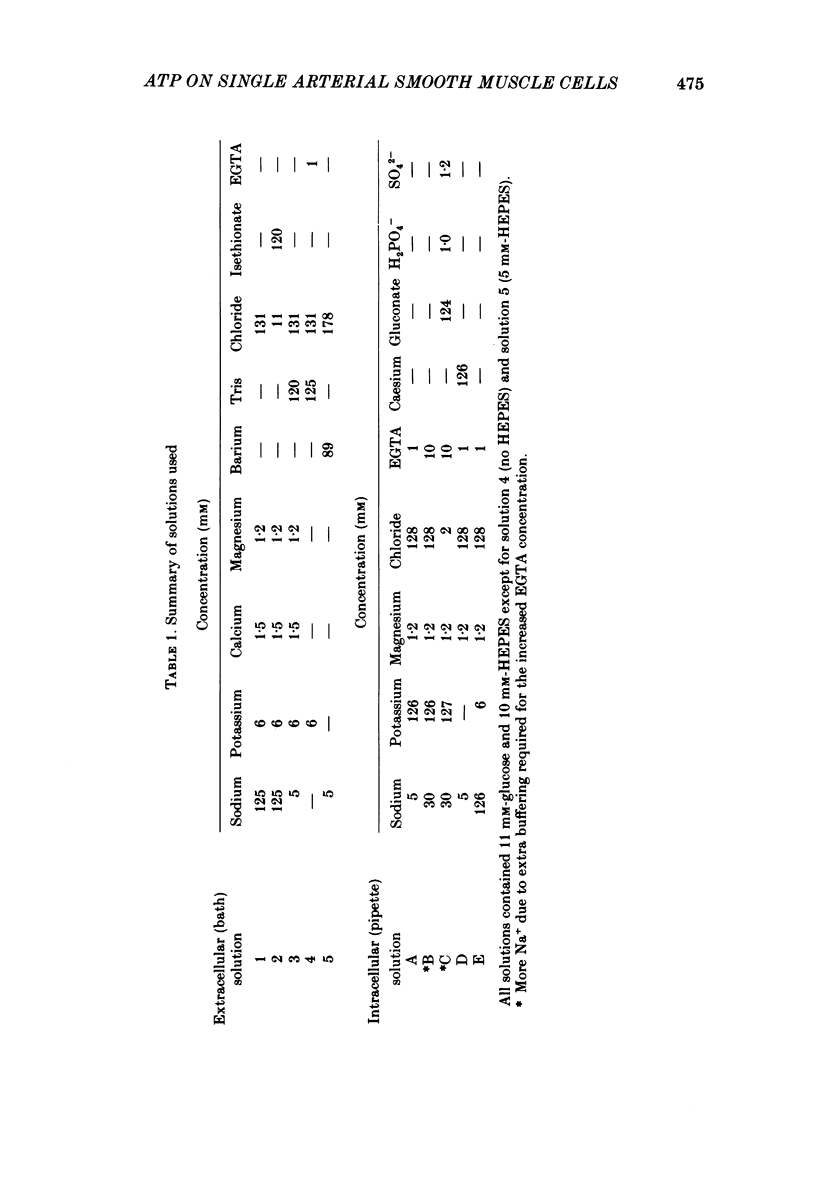
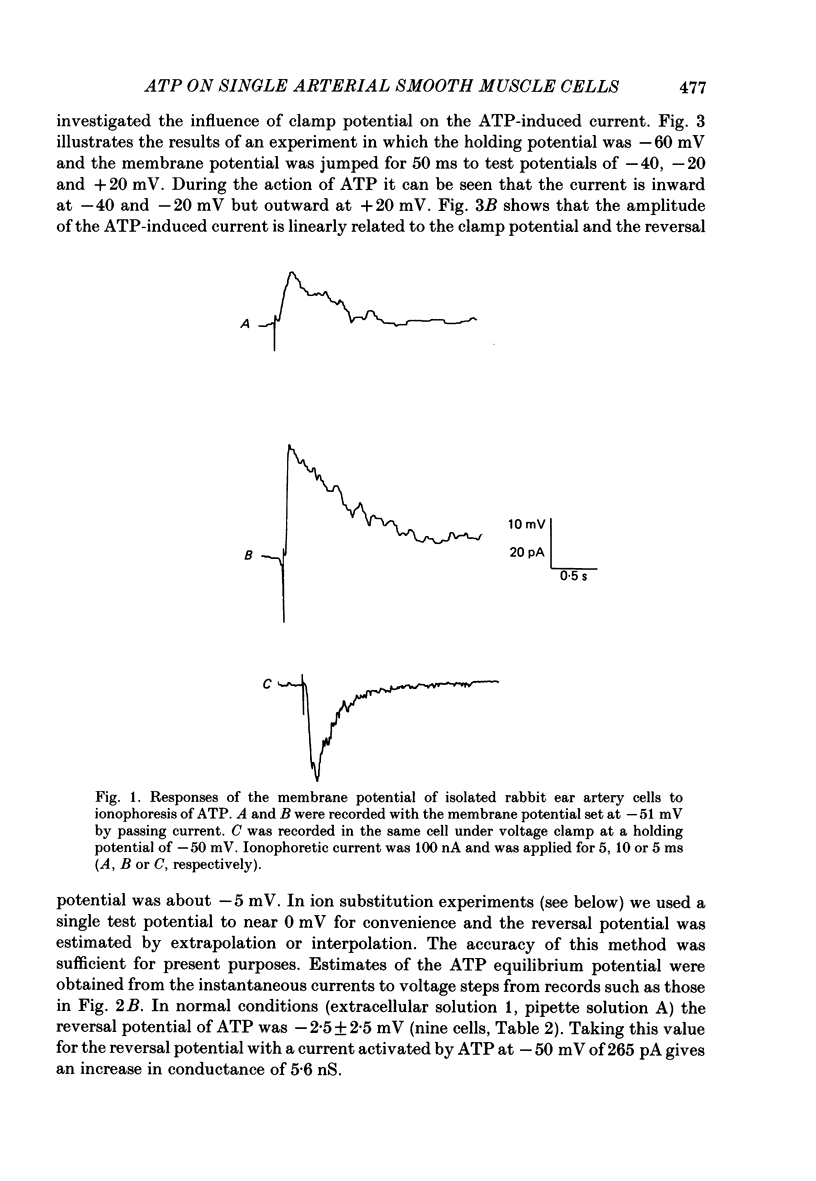
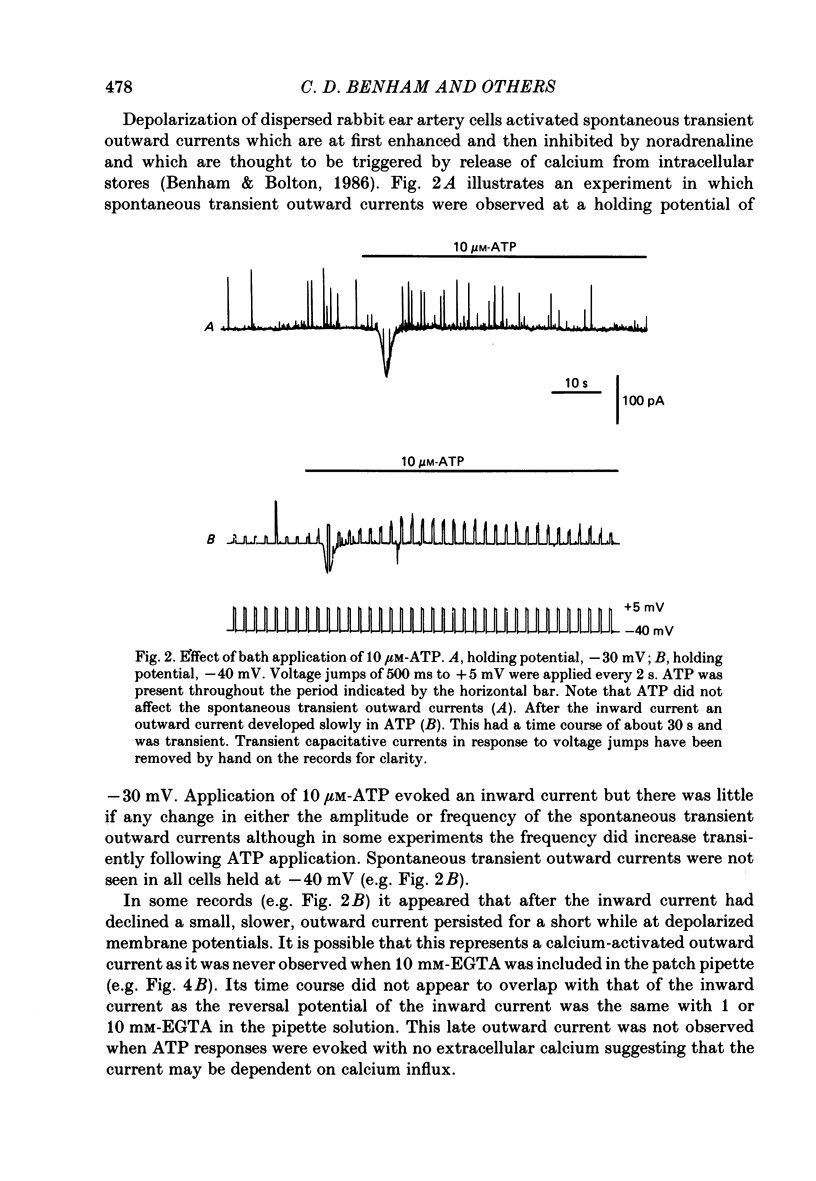
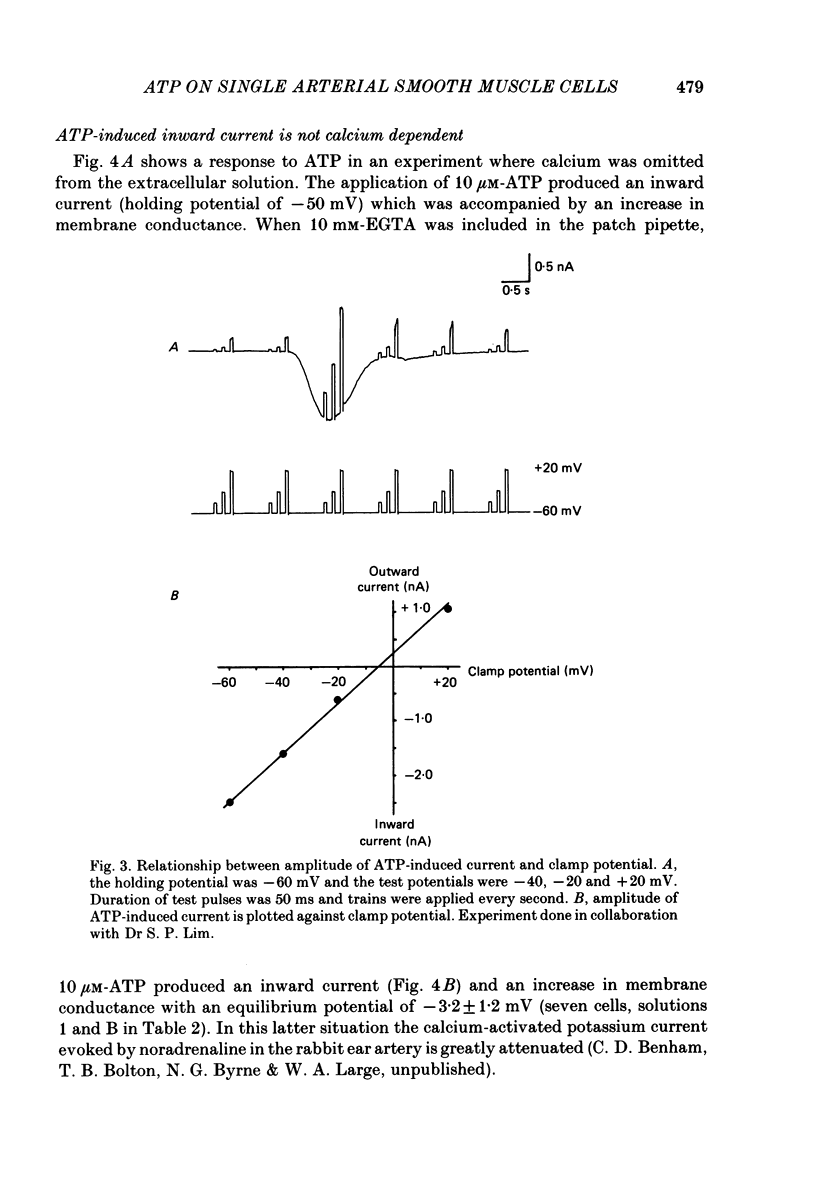
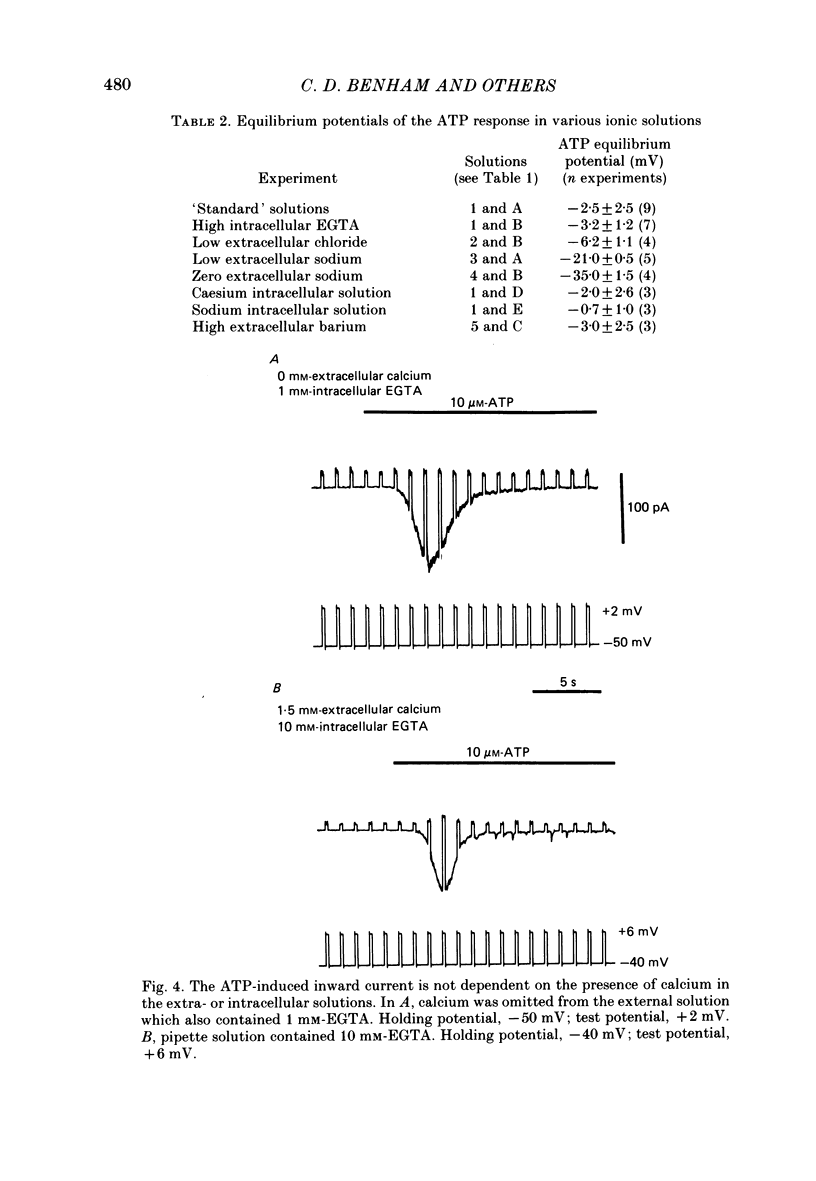
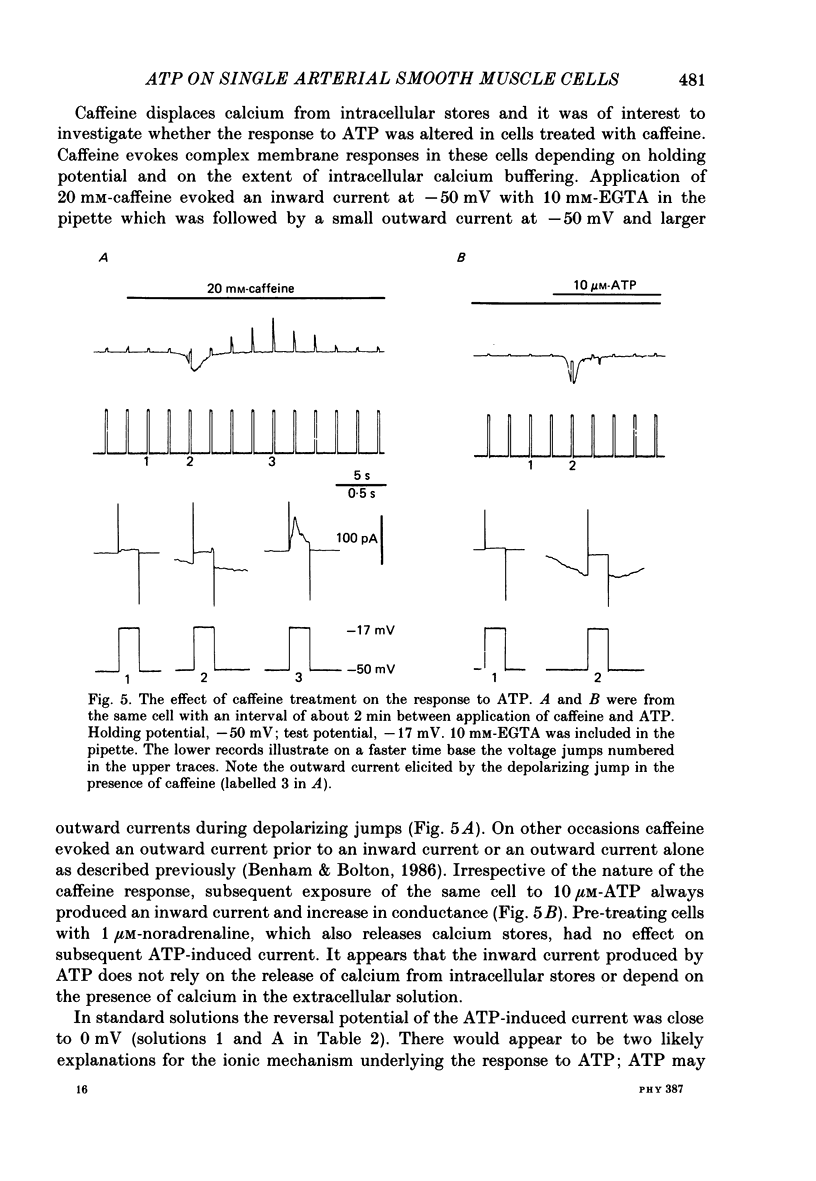
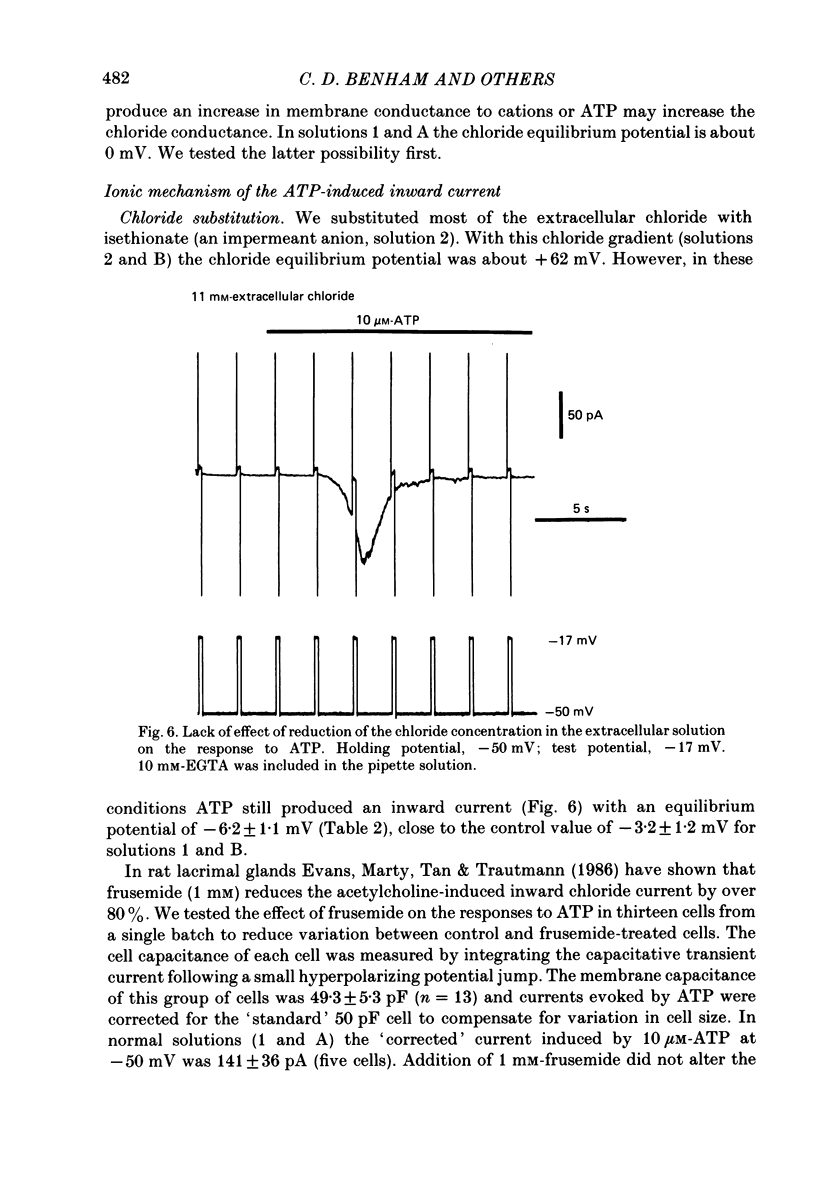
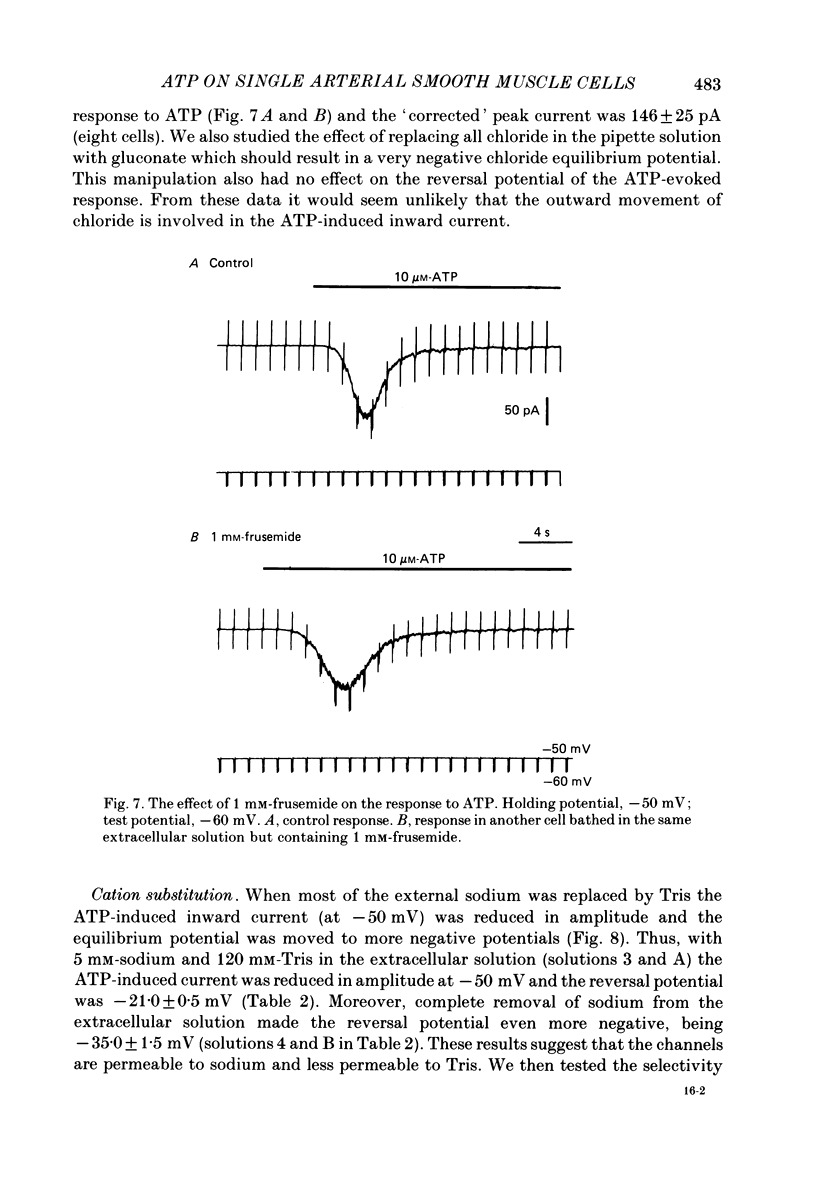
1. Adenosine triphosphate (ATP), applied in the bathing solution or ionophoretically, depolarized freshly dispersed single arterial smooth muscle cells obtained by collagenase and elastase treatment of the rabbit ear artery. 2. Ionophoretic application of ATP evoked an inward current with a latency of about 70 ms and a time to peak of about 230 ms in cells held under voltage clamp using whole-cell patch-pipette techniques. 3. Bath application of 10 microM-ATP evoked a transient inward current at negative holding potentials. The amplitude of the ATP-induced current was linearly related to the clamp potential with a reversal potential near 0 mV. Removal of extracellular calcium, buffering intracellular calcium with high EGTA concentration, or depleting calcium stores with caffeine or noradrenaline treatment did not affect the ATP-evoked current. 4. Changing the chloride concentration gradient by decreasing extracellular or intracellular chloride concentration, or using the chloride channel blocker, frusemide, had no effect on the currents. 5. Replacing sodium with Tris shifted the reversal potential to more negative potentials. The reversal potential was not affected by exchanging intracellular potassium for caesium or sodium. Replacing extracellular sodium with 89 mM-barium also had little effect on the reversal potential. 6. These results are consistent with ATP activating a conductance that is cation selective but allows both monovalent and divalent cations to pass across the membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford O. S., Dawes G. S., Ward R. A. Effects of apomorphine and haloperidol in fetal lambs. J Physiol. 1986 Aug;377:37–47. doi: 10.1113/jphysiol.1986.sp016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Comparison of the biphasic excitatory junction potential with membrane responses to adenosine triphosphate and noradrenaline in the rat anococcygeus muscle. Br J Pharmacol. 1984 Nov;83(3):751–758. doi: 10.1111/j.1476-5381.1984.tb16229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A., Tan Y. P., Trautmann A. Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflugers Arch. 1986 Jan;406(1):65–68. doi: 10.1007/BF00582955. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O., Silverberg G. D. Noradrenaline receptors on the rat basilar artery. J Physiol. 1982 Jul;328:351–360. doi: 10.1113/jphysiol.1982.sp014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A. Membrane potential responses of the mouse anococcygeus muscle to ionophoretically applied noradrenaline. J Physiol. 1982 May;326:385–400. doi: 10.1113/jphysiol.1982.sp014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I., Fujiwara M., Miura A., Sakakibara Y. Possible involvement of adenine nucleotides in sympathetic neuroeffector mechanisms of dog basilar artery. J Pharmacol Exp Ther. 1981 Feb;216(2):401–409. [PubMed] [Google Scholar]
- Ritchie A. K., Fambrough D. M. Ionic properties of the acetylcholine receptor in cultured rat myotubes. J Gen Physiol. 1975 Jun;65(6):751–767. doi: 10.1085/jgp.65.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Electrical responses of smooth muscle cells of the rabbit ear artery to adenosine triphosphate. J Physiol. 1985 Feb;359:401–415. doi: 10.1113/jphysiol.1985.sp015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kou K. Electrical components contributing to the nerve-mediated contractions in the smooth muscles of the rabbit ear artery. Jpn J Physiol. 1983;33(5):743–756. doi: 10.2170/jjphysiol.33.743. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


