Abstract
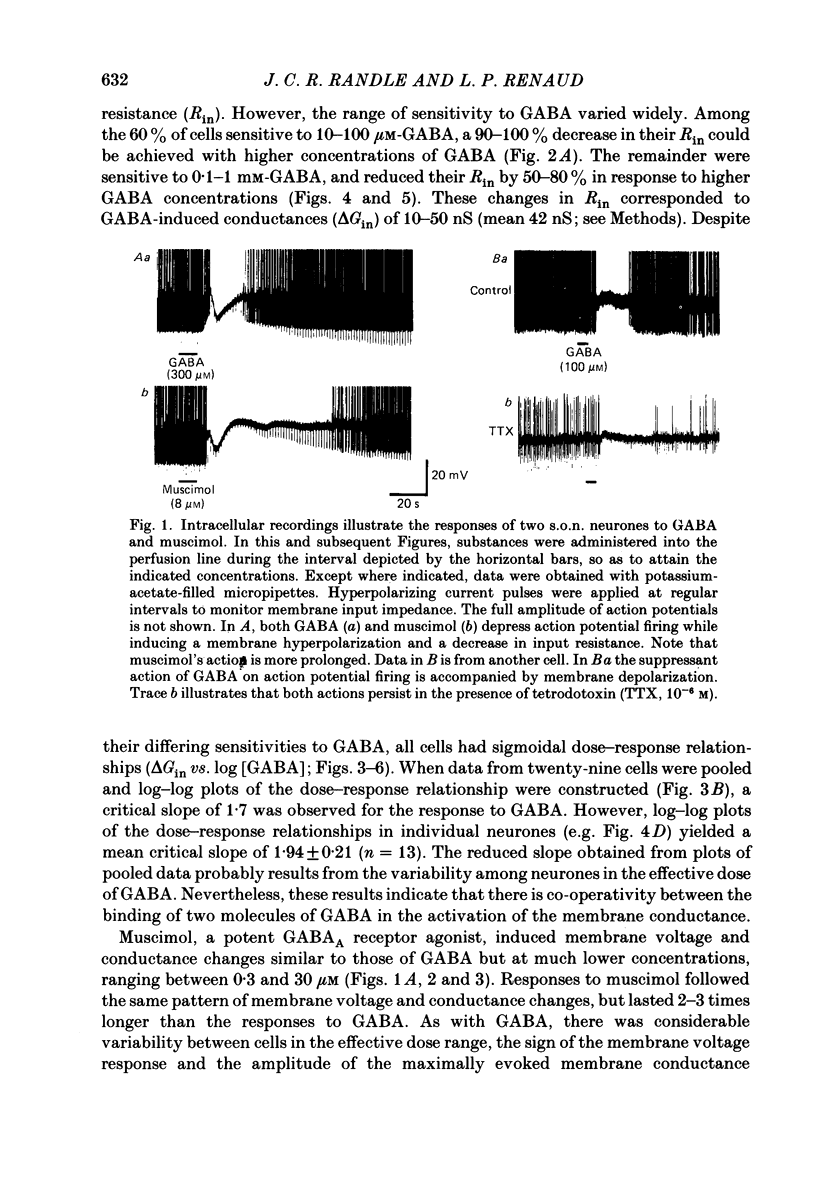
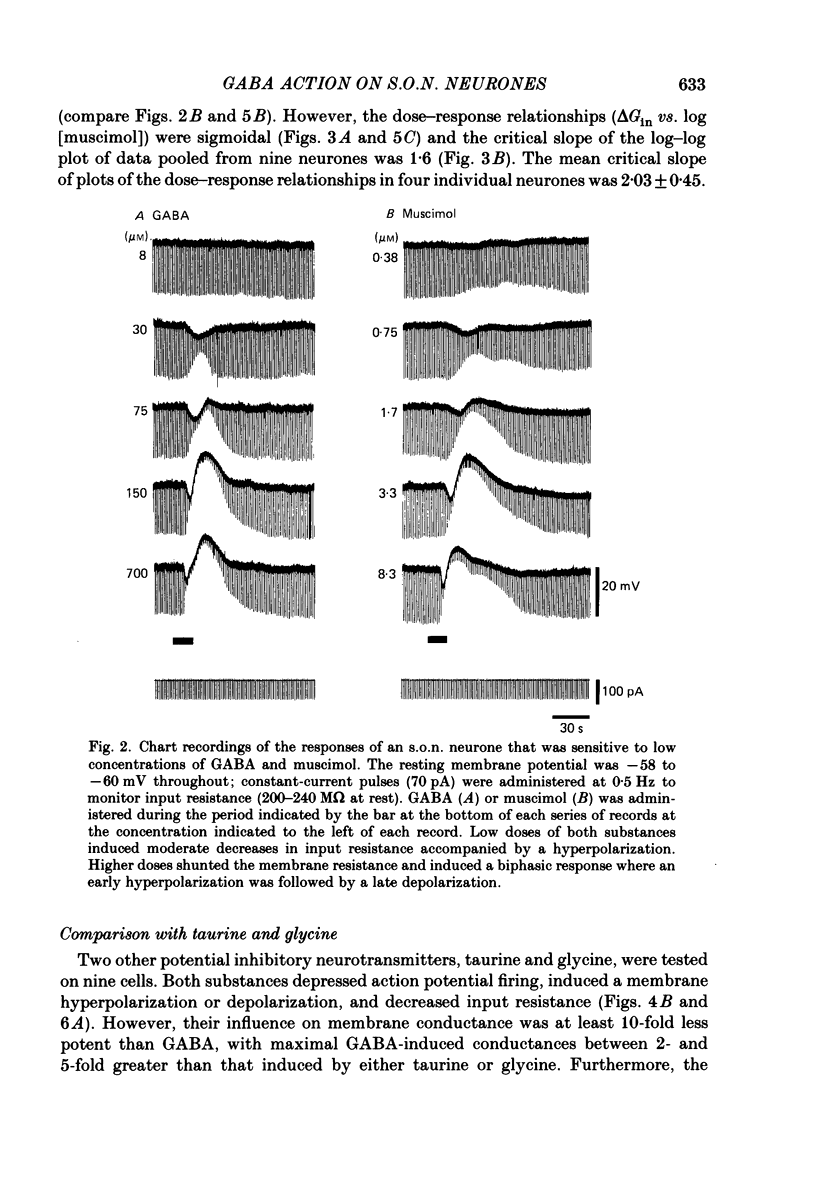
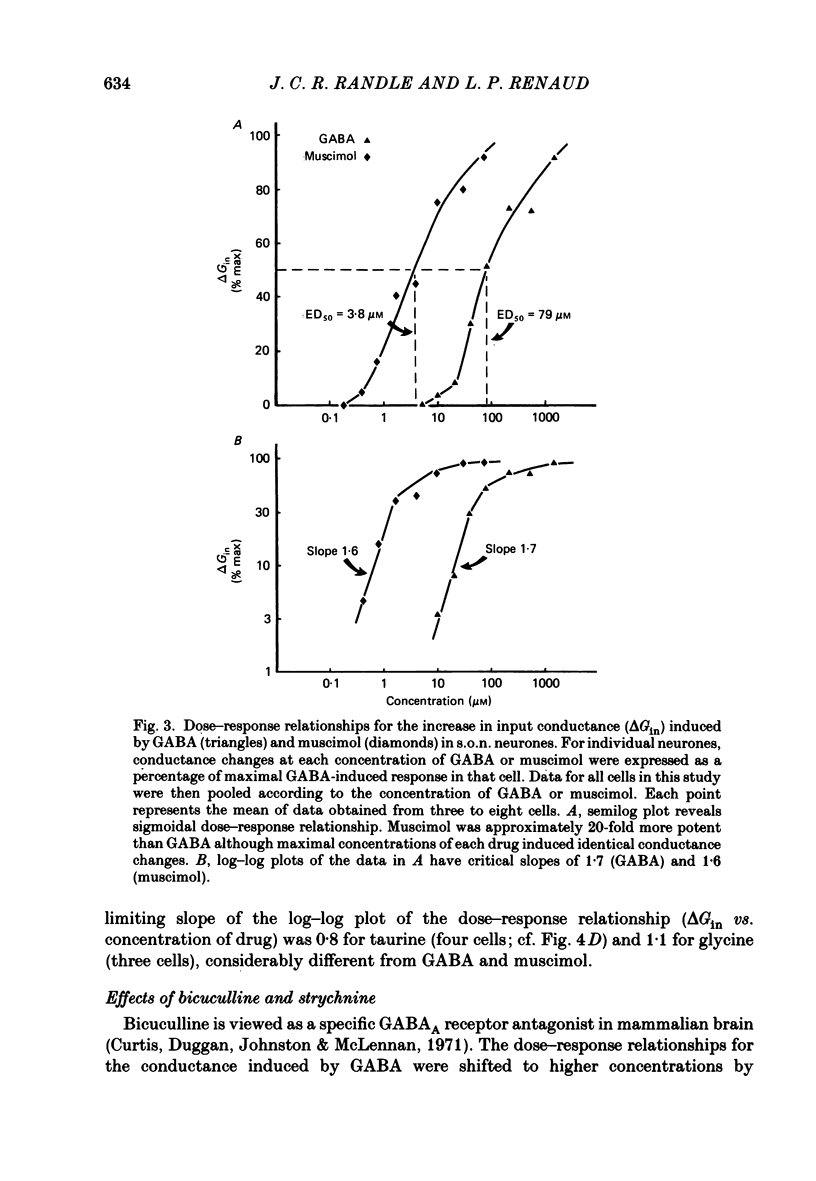
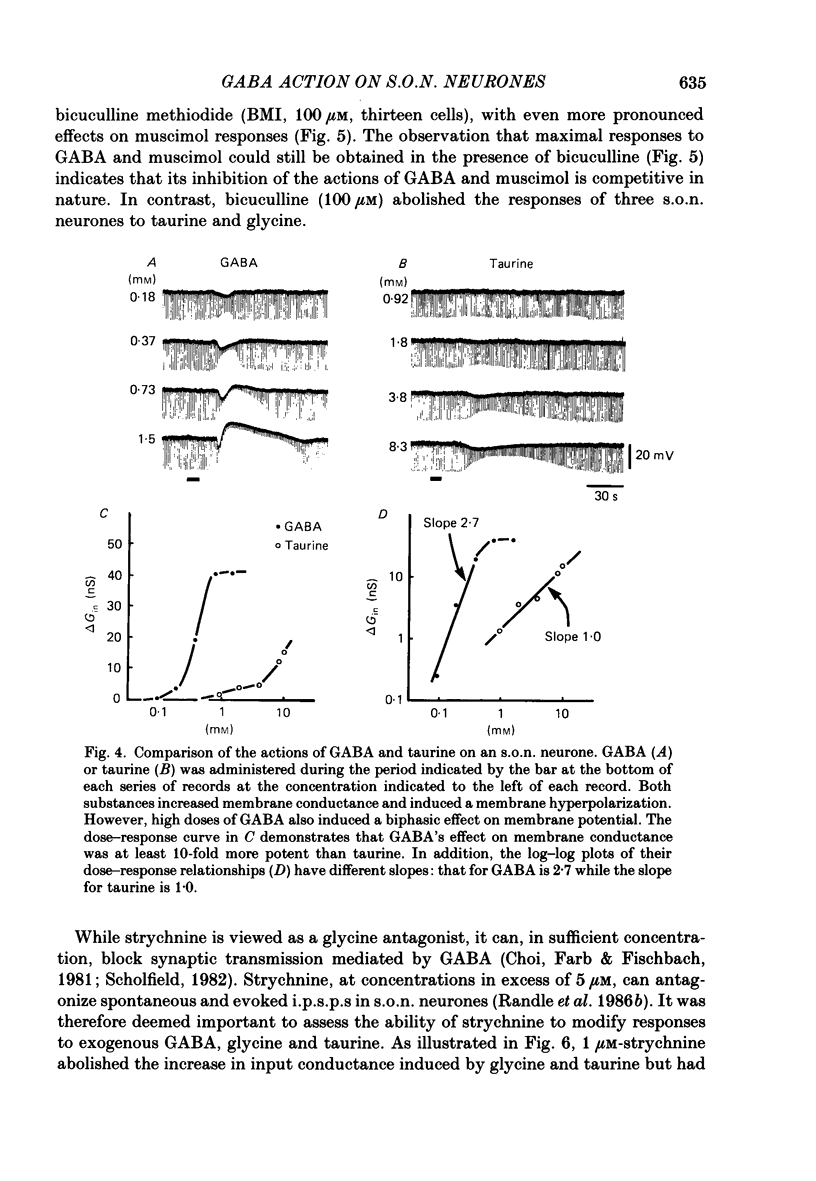
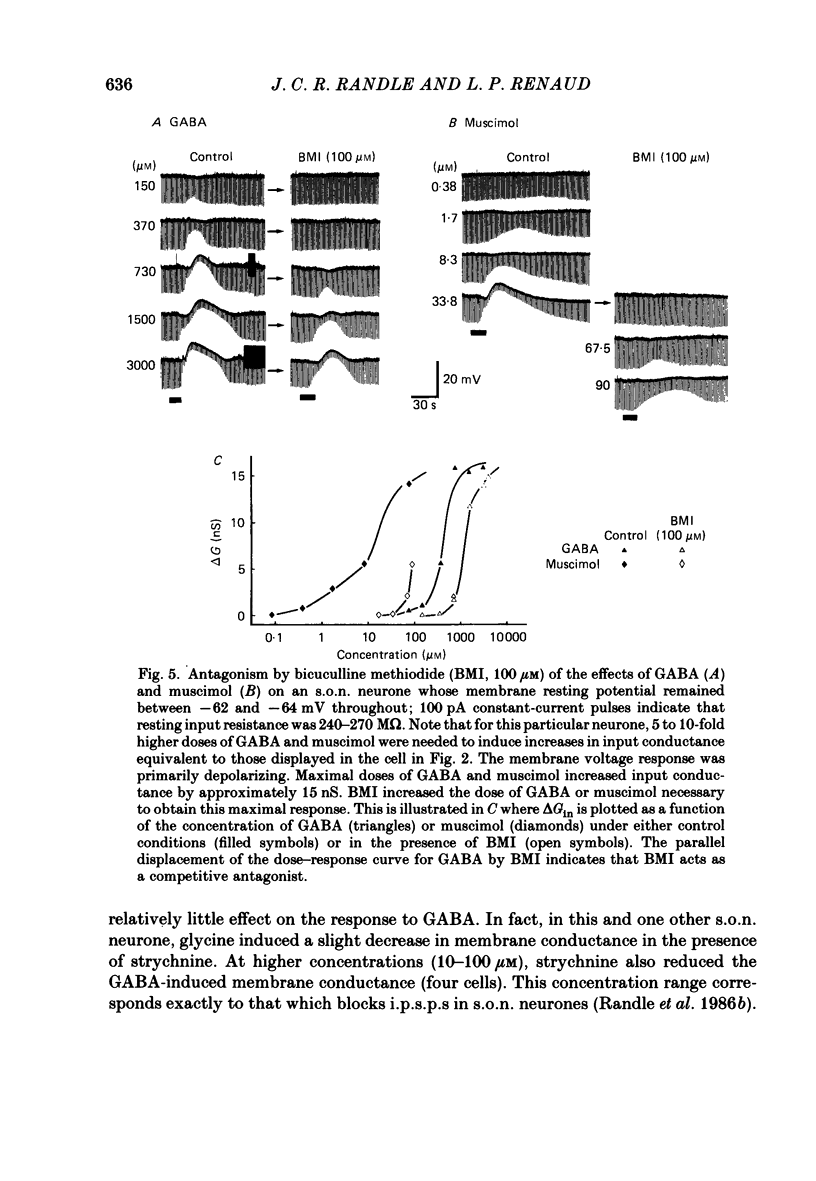
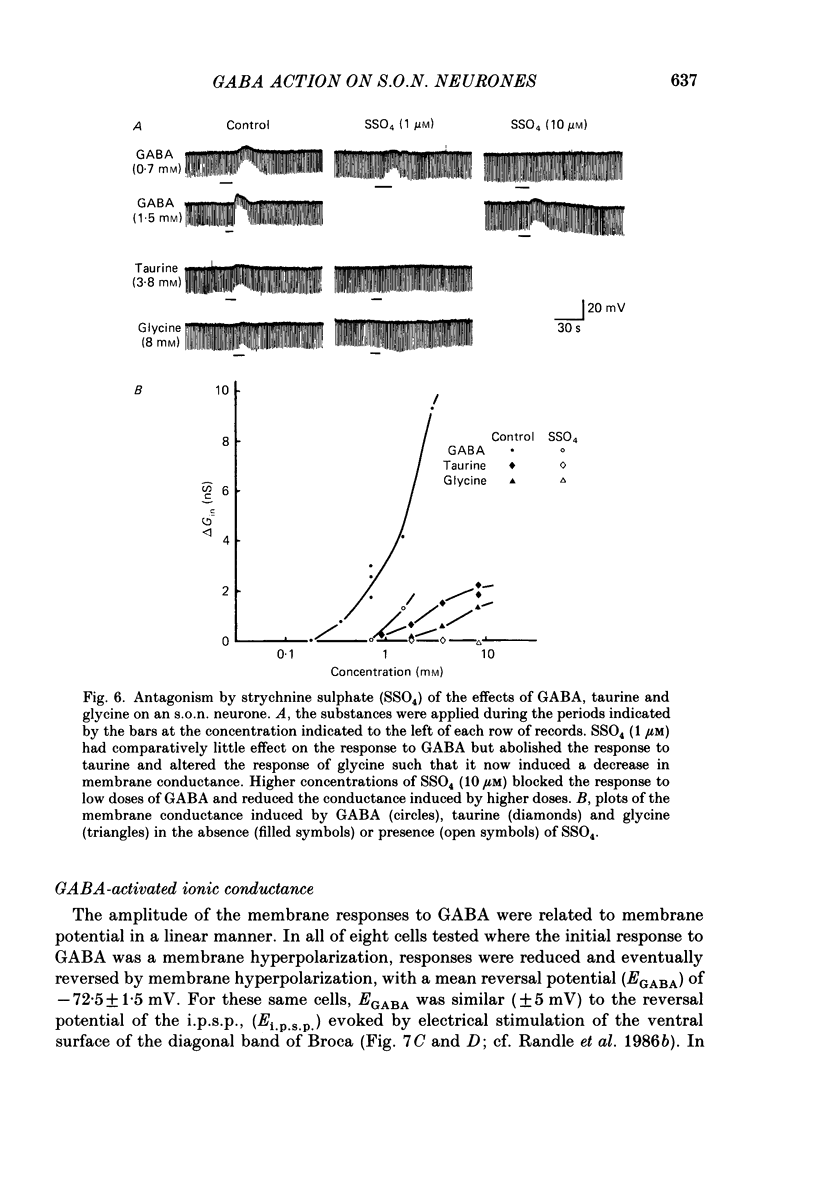
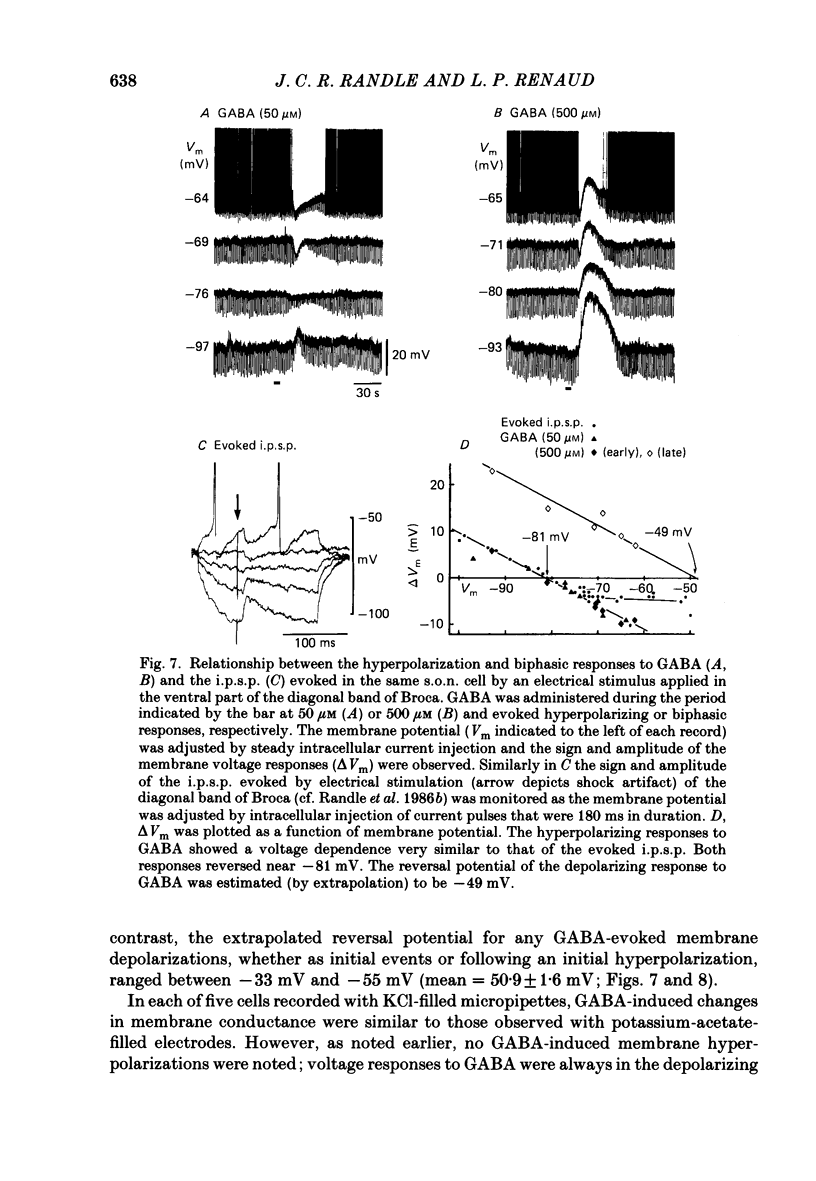
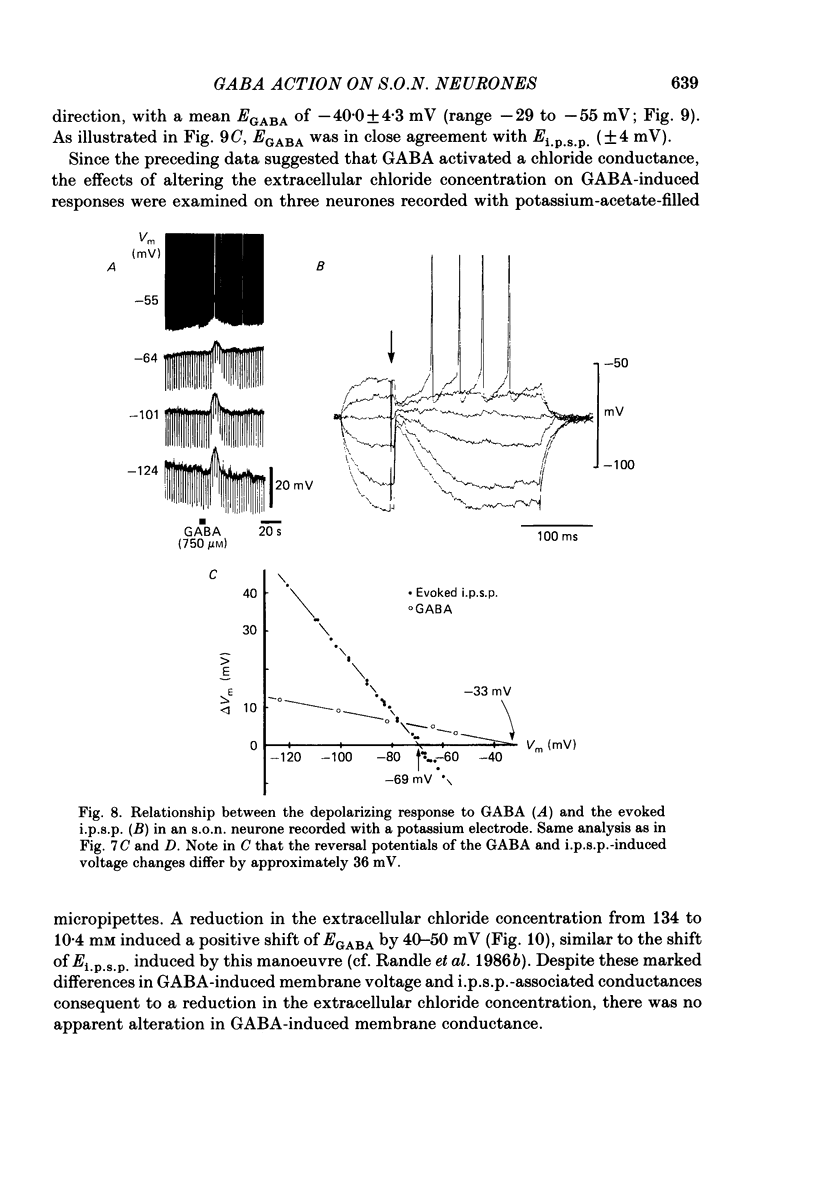
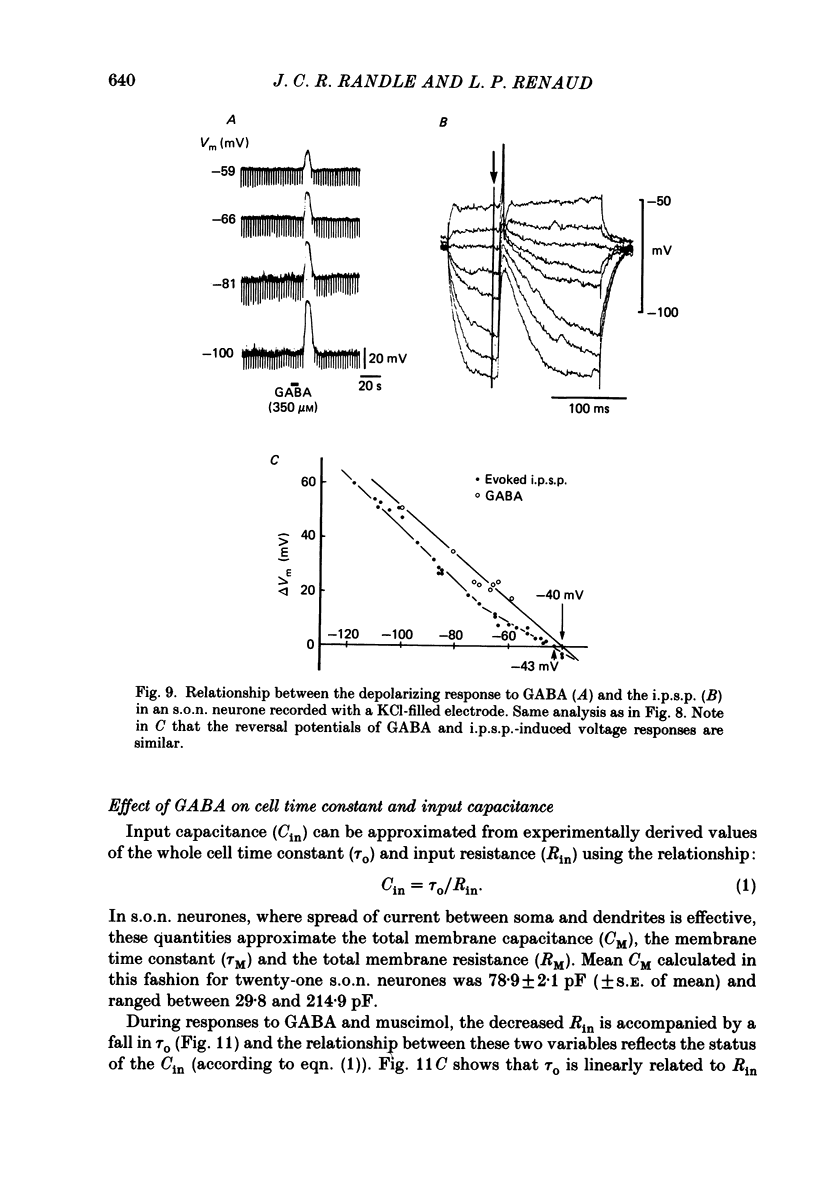
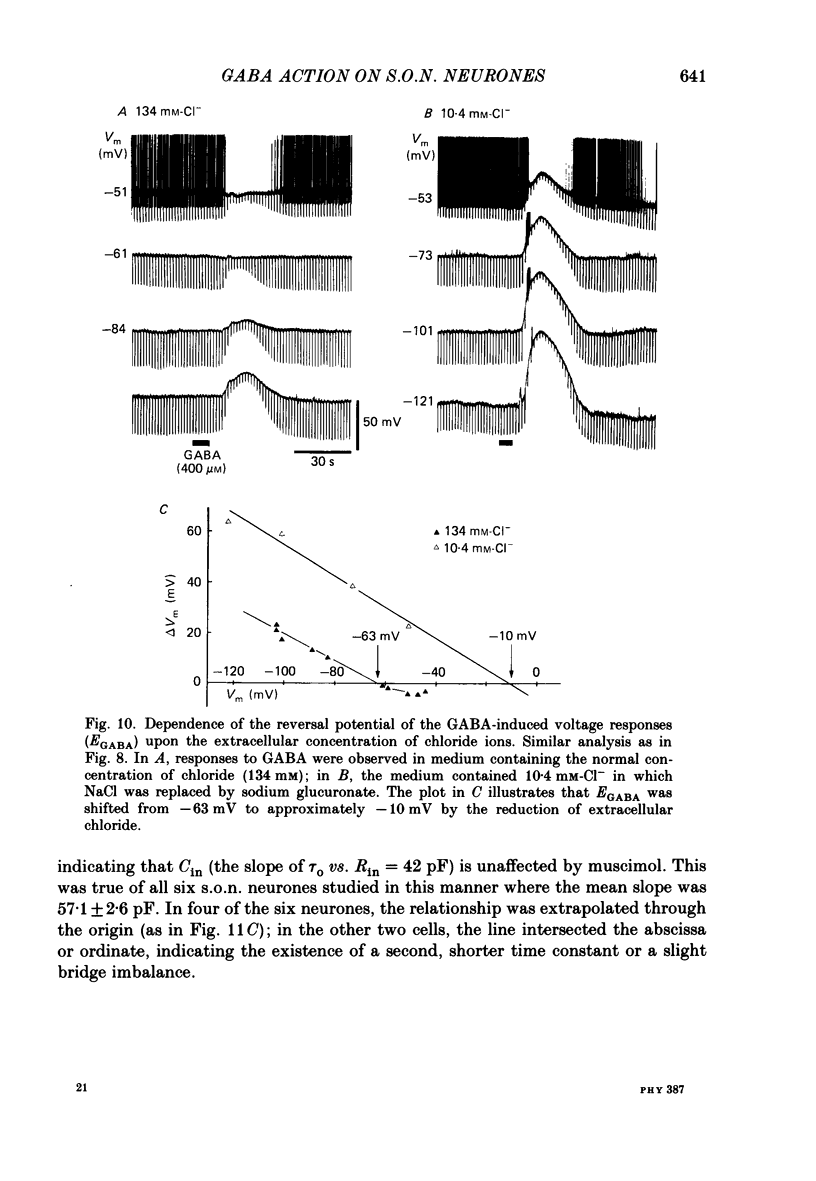
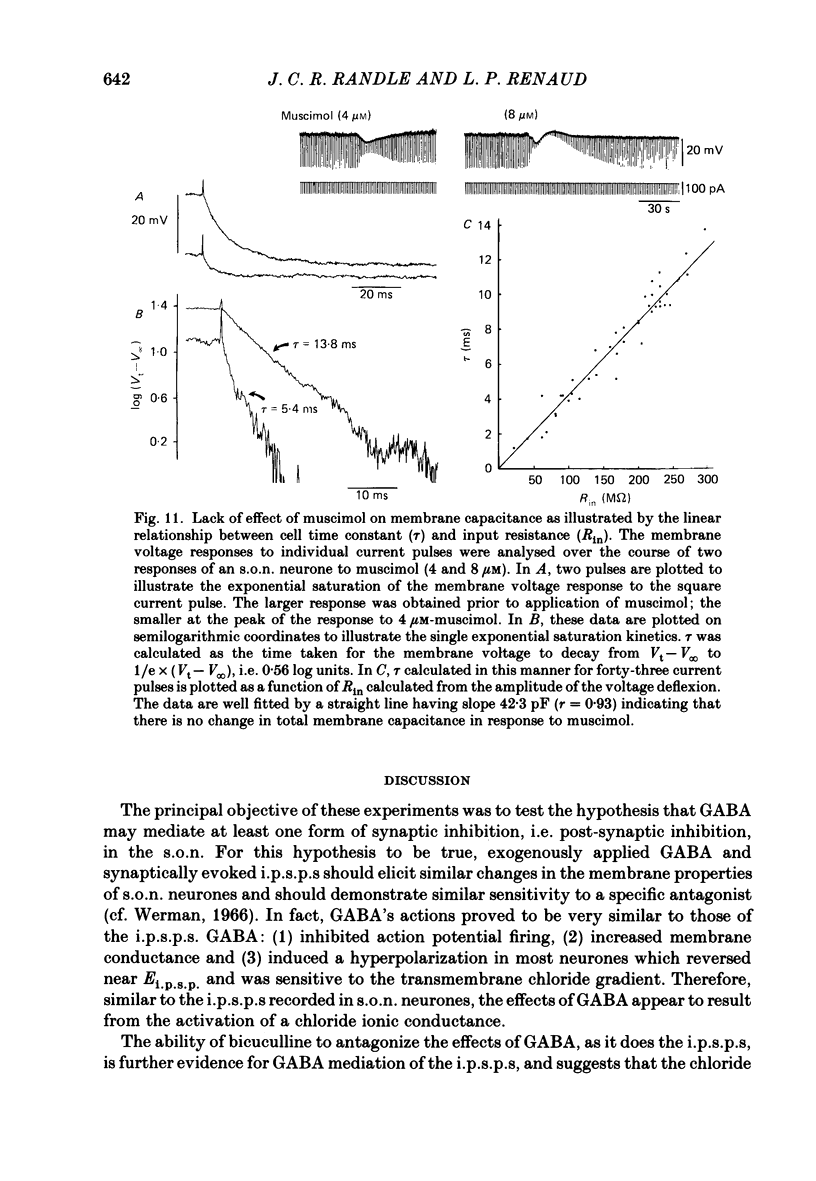
1. Intracellular recordings were obtained from thirty-eight rat supraoptic nucleus (s.o.n.) neurosecretory neurones in perfused hypothalamic explants. Changes in membrane potential and conductance were monitored following application of gamma-aminobutyric acid (GABA), and related agonists and antagonists. 2. GABA depressed action potential discharge of all of thirty-five s.o.n. neurones tested and induced either membrane hyperpolarization or depolarization. Neurones that displayed membrane hyperpolarization in response to lower GABA concentrations (30-300 microM) demonstrated a biphasic membrane voltage change with a later depolarizing phase as a response to higher concentrations (up to 3000 microM). 3. GABA (10-3000 microM) induced a prominent concentration-dependent increase in membrane conductance in all neurones. The critical slope for the log-log plot of [GABA] vs. GABA-induced membrane conductance was 1.7, indicating co-operativity in the GABA receptor-induced conductance change. 4. Muscimol (0.3-30 microM) potently mimicked all the effects of GABA. Bicuculline (1-100 microM) antagonized the effects of GABA and muscimol in a competitive manner. 5. Glycine and taurine (1-10 mM) had weak effects, although comparatively similar to those of GABA. These actions were blocked both by bicuculline (100 microM) and by strychnine (1 microM). At higher concentrations (greater than 10 microM), strychnine also antagonized the actions of GABA. 6. In recordings with potassium-acetate-filled micropipettes, the reversal potential of hyperpolarizing membrane voltage responses to GABA was -72.5 +/- 1.5 mV in close agreement (+/- 5 mV) with the reversal potential of inhibitory post-synaptic potentials (i.p.s.p.s) recorded in the same neurones. Depolarizing responses to GABA reversed polarity at -50 +/- 1.6 mV. In recordings with KCl-filled micropipettes, voltage responses to GABA were always depolarizing and reversed near -40.0 +/- 4.3 mV. Similarly, reduction of the concentration of chloride ions in the perfusion medium from 134 to 10.4 mM induced a positive shift of the GABA reversal potential by 40-50 mV. 7. From measurements of input resistance (Rin) and cell time constant (tau O), input capacitance (Cin; representing total membrane capacitance) was calculated as 78.9 +/- 2.1 pF. During responses to GABA or muscimol, decreased Rin was accompanied by a linearly related decrease in tau o indicating that these substances had no effect on the membrane capacitance of s.o.n. neurones.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983 Sep 9;221(4615):1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont K., Chilton W. S., Yamamura H. I., Enna S. J. Muscimol binding in rat brain: association with synaptic GABA receptors. Brain Res. 1978 Jun 9;148(1):153–162. doi: 10.1016/0006-8993(78)90385-2. [DOI] [PubMed] [Google Scholar]
- Bioulac B., Gaffori O., Harris M., Vincent J. D. Effects of acetylcholine, sodium glutamate and GABA on the discharge of supraoptic neurons in the rat. Brain Res. 1978 Oct 6;154(1):159–162. doi: 10.1016/0006-8993(78)91064-8. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985 Dec;54(6):1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Non-synaptic depolarizing potentials in rat supraoptic neurones recorded in vitro. J Physiol. 1986 Jul;376:493–505. doi: 10.1113/jphysiol.1986.sp016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. A perfused in vitro preparation of hypothalamus for electrophysiological studies on neurosecretory neurons. J Neurosci Methods. 1983 Mar;7(3):203–214. doi: 10.1016/0165-0270(83)90002-x. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T. J., Haywood J. R. GABAergic inhibition of hypertonic saline-induced vasopressin-dependent hypertension. J Pharmacol Exp Ther. 1985 Jun;233(3):663–671. [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Responses of the guinea-pig isolated olfactory cortex slice to gamma-aminobutyric acid recorded with extracellular electrodes. Br J Pharmacol. 1979 Feb;65(2):347–353. doi: 10.1111/j.1476-5381.1979.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. GABA-mediated synaptic potentials in chick spinal cord and sensory neurons. J Neurophysiol. 1981 Apr;45(4):632–643. doi: 10.1152/jn.1981.45.4.632. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Fischbach G. D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- Cirino M., Renaud L. P. Influence of lateral septum and amygdala stimulation on the excitability of hypothalamic supraoptic neurons. An electrophysiological study in the rat. Brain Res. 1985 Feb 11;326(2):357–361. doi: 10.1016/0006-8993(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., McLennan H. Antagonism between bicuculline and GABA in the cat brain. Brain Res. 1971 Oct 8;33(1):57–73. doi: 10.1016/0006-8993(71)90305-2. [DOI] [PubMed] [Google Scholar]
- Enna S. J., Gallagher J. P. Biochemical and electrophysiological characteristics of mammalian GABA receptors. Int Rev Neurobiol. 1983;24:181–212. doi: 10.1016/s0074-7742(08)60222-6. [DOI] [PubMed] [Google Scholar]
- Enna S. J., Snyder S. H. GABA receptor binding in frog spinal cord and brain. J Neurochem. 1977 Apr;28(4):857–860. doi: 10.1111/j.1471-4159.1977.tb10639.x. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Rocha e Silva M., Jr Inhibition of vasopressin release to carotid occlusion by gamma-aminobutyric acid and glycine. Br J Pharmacol. 1981 Jan;72(1):17–24. doi: 10.1111/j.1476-5381.1981.tb09099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Harris M. C. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979 Jul;82(1):115–125. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- Homma S., Rovainen C. M. Conductance increases produced by glycine and gamma-aminobutyric acid in lamprey interneurones. J Physiol. 1978 Jun;279:231–252. doi: 10.1113/jphysiol.1978.sp012342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Jhamandas J. H., Renaud L. P. A gamma-aminobutyric-acid-mediated baroreceptor input to supraoptic vasopressin neurones in the rat. J Physiol. 1986 Dec;381:595–606. doi: 10.1113/jphysiol.1986.sp016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Lodge D. Muscimol uptake, release and binding in rat brain slices. J Neurochem. 1978 Dec;31(6):1519–1523. doi: 10.1111/j.1471-4159.1978.tb06579.x. [DOI] [PubMed] [Google Scholar]
- Knepel W., Nutto D., Hertting G. Evidence for the involvement of a GABA-mediated inhibition in the hypovolaemia-induced vasopressin release. Pflugers Arch. 1980 Nov;388(2):177–183. doi: 10.1007/BF00584125. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. GABA and glycine actions on spinal motoneurons. Can J Physiol Pharmacol. 1977 Jun;55(3):658–669. doi: 10.1139/y77-090. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980 Sep 11;287(5778):154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. GABA and muscimol open ion channels of different lifetimes on cultured mouse spinal cord cells. Brain Res. 1981 Jan 5;204(1):242–247. doi: 10.1016/0006-8993(81)90672-7. [DOI] [PubMed] [Google Scholar]
- Meyer D. K., Oertel W. H., Brownstein M. J. Deafferentation studies on the glutamic acid decarboxylase content of the supraoptic nucleus of the rat. Brain Res. 1980 Oct 27;200(1):165–168. doi: 10.1016/0006-8993(80)91102-6. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. Properties of gamma-aminobutyric acid receptor binding with (+)-[3H]bicuculline methiodide in rat cerebellum. Mol Pharmacol. 1978 Mar;14(2):256–265. [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. The pharmacology of recurrent inhibition in the supraoptic neurosecretory system. Brain Res. 1971 Dec 24;35(2):501–511. doi: 10.1016/0006-8993(71)90491-4. [DOI] [PubMed] [Google Scholar]
- Pickles H. G. Presynaptic gamma-aminobutyric acid responses in the olfactory cortex. Br J Pharmacol. 1979 Feb;65(2):223–228. doi: 10.1111/j.1476-5381.1979.tb07822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Pérez de la Mora M., Possani L. D., Tapia R., Teran L., Palacios R., Fuxe K., Hökfelt T., Ljungdahl A. Demonstration of central gamma-aminobutyrate-containing nerve terminals by means of antibodies against glutamate decarboxylase. Neuroscience. 1981;6(5):875–895. doi: 10.1016/0306-4522(81)90169-x. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Serial reconstruction of Lucifer yellow-labeled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience. 1986 Feb;17(2):453–467. doi: 10.1016/0306-4522(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Sakai K. K., Marks B. H., George J. M., Koestner A. The isolated organ-cultured supraoptic nucleus as a neuropharmacological test system. J Pharmacol Exp Ther. 1974 Sep;190(3):482–491. [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. Responses to gamma-aminobutyric acid applied to cell bodies and dendrites of rat visual cortical neurons. Brain Res. 1985 Dec 9;358(1-2):385–389. doi: 10.1016/0006-8993(85)90990-4. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Antagonism of gamma-aminobutyric acid and muscimol by picrotoxin, bicuculline, strychnine, bemegride, leptazol, D-tubocurarine and theophylline in the isolated olfactory cortex. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):274–280. doi: 10.1007/BF00501165. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Evidence that bicuculline and picrotoxin act at separate sites to antagonize gamma-aminobutyric acid in rat cuneate nucleus. Neuropharmacology. 1980 Jan;19(1):39–45. doi: 10.1016/0028-3908(80)90164-1. [DOI] [PubMed] [Google Scholar]
- Snodgrass S. R. Use of 3H-muscimol for GABA receptor studies. Nature. 1978 Jun 1;273(5661):392–394. doi: 10.1038/273392a0. [DOI] [PubMed] [Google Scholar]
- Tappaz M. L., Brownstein M. J., Kopin I. J. Glutamate decarboxylase (GAD) and gamma-aminobutyric acid (GABA) in discrete nuclei of hypothalamus and substantia nigra. Brain Res. 1977 Apr 8;125(1):109–121. doi: 10.1016/0006-8993(77)90363-8. [DOI] [PubMed] [Google Scholar]
- Tappaz M. L., Wassef M., Oertel W. H., Paut L., Pujol J. F. Light- and electron-microscopic immunocytochemistry of glutamic acid decarboxylase (GAD) in the basal hypothalamus: morphological evidence for neuroendocrine gamma-aminobutyrate (GABA). Neuroscience. 1983 Jun;9(2):271–287. doi: 10.1016/0306-4522(83)90293-2. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Paut L., Tappaz M. L. Immunocytochemical analysis of the GABAergic innervation of oxytocin- and vasopressin-secreting neurons in the rat supraoptic nucleus. Neuroscience. 1986 Sep;19(1):207–222. doi: 10.1016/0306-4522(86)90016-3. [DOI] [PubMed] [Google Scholar]
- Werman R. Criteria for identification of a central nervous system transmitter. Comp Biochem Physiol. 1966 Aug;18(4):745–766. doi: 10.1016/0010-406x(66)90209-x. [DOI] [PubMed] [Google Scholar]
- Wible J. H., Jr, Zerbe R. L., DiMicco J. A. Stimulation of vasopressin release by gamma-aminobutyric acid antagonists in spinal cord transected rats. J Pharmacol Exp Ther. 1985 Aug;234(2):378–385. [PubMed] [Google Scholar]
- Williams M., Risley E. A. Characterization of the binding of [3H]muscimol, a potent gamma-aminobutyric acid agonist, to rat brain synaptosomal membranes using a filtration assay. J Neurochem. 1979 Mar;32(3):713–718. doi: 10.1111/j.1471-4159.1979.tb04553.x. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N. Dual ultrastructural localization of two neurotransmitter-related antigens: colloidal gold-labeled neurophysin-immunoreactive supraoptic neurons receive peroxidase-labeled glutamate decarboxylase- or gold-labeled GABA-immunoreactive synapses. J Neurosci. 1985 Nov;5(11):2940–2954. doi: 10.1523/JNEUROSCI.05-11-02940.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


