Abstract
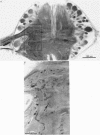
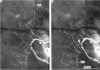
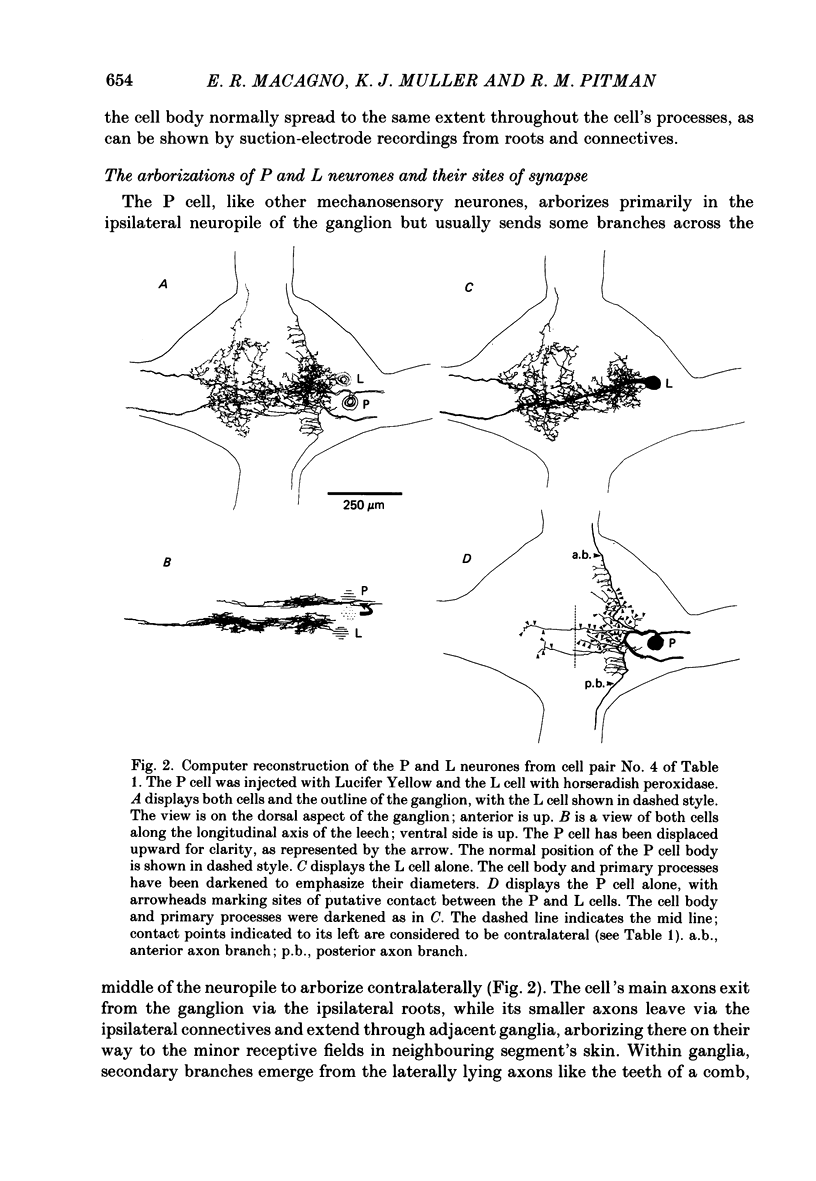
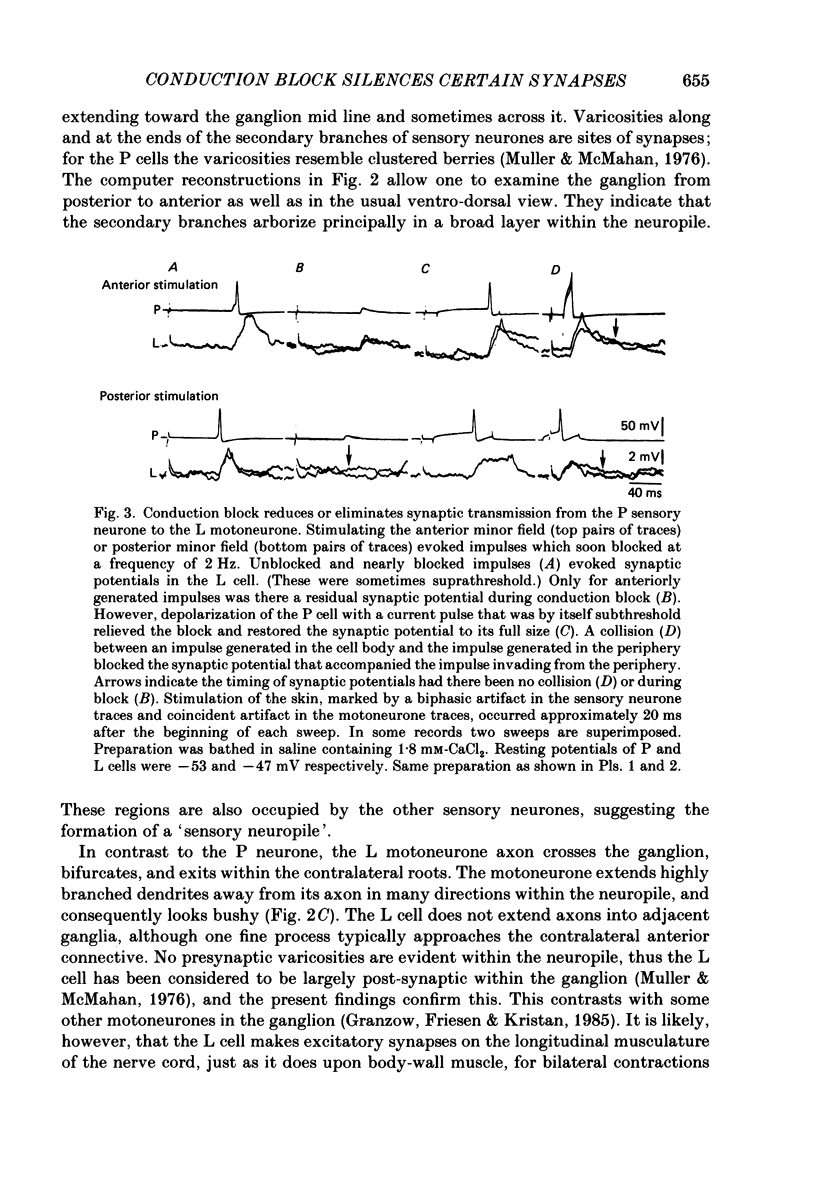
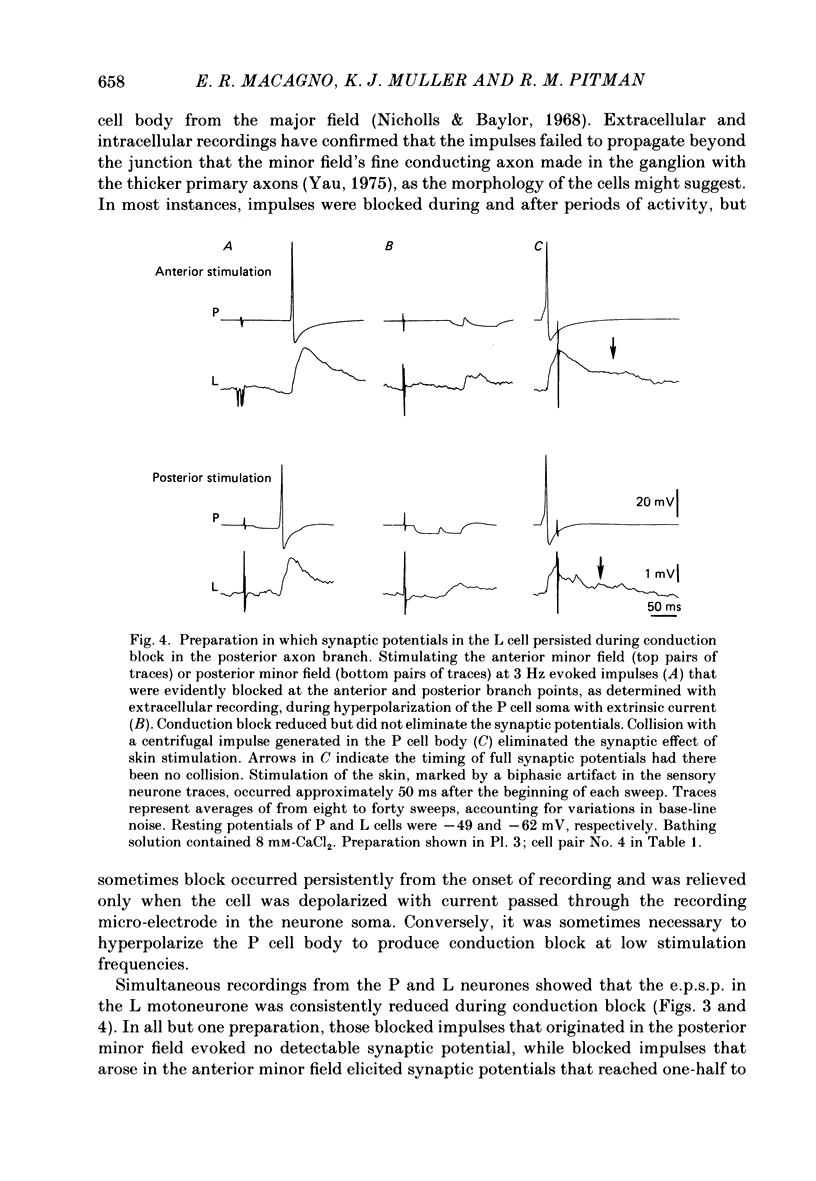
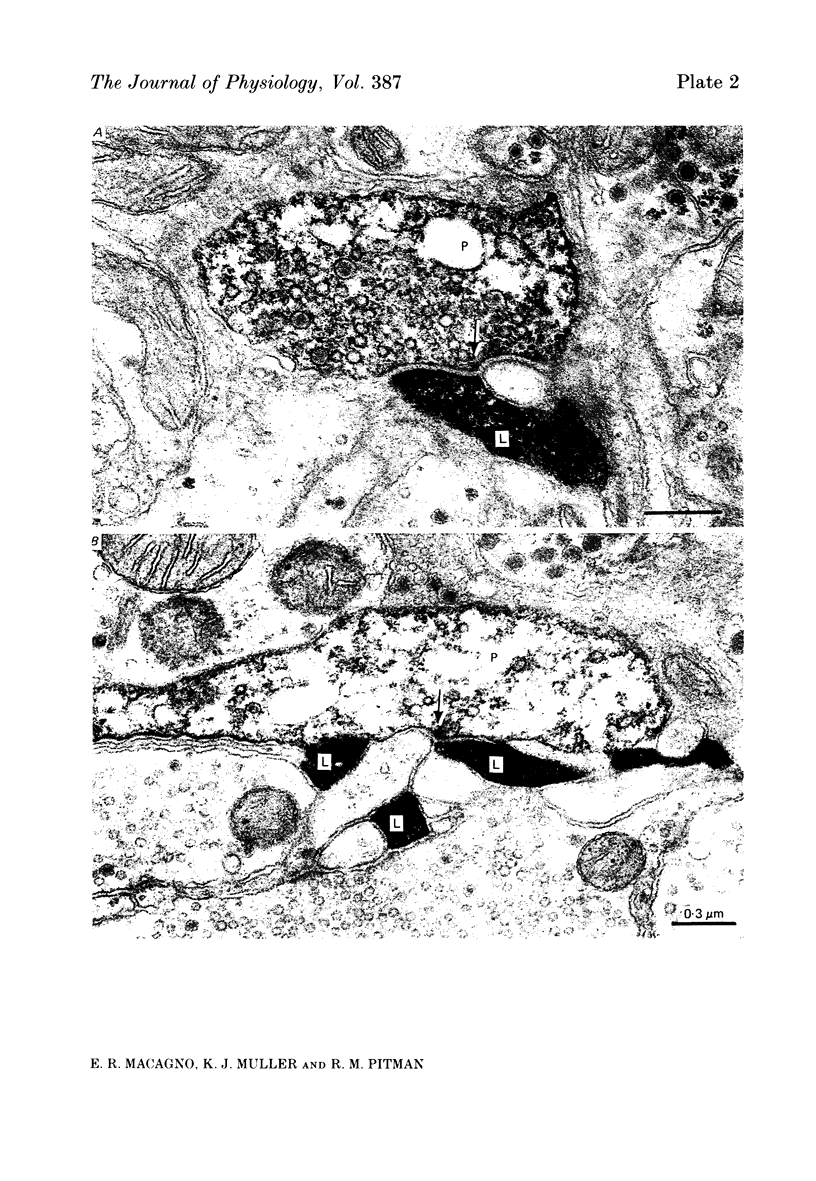
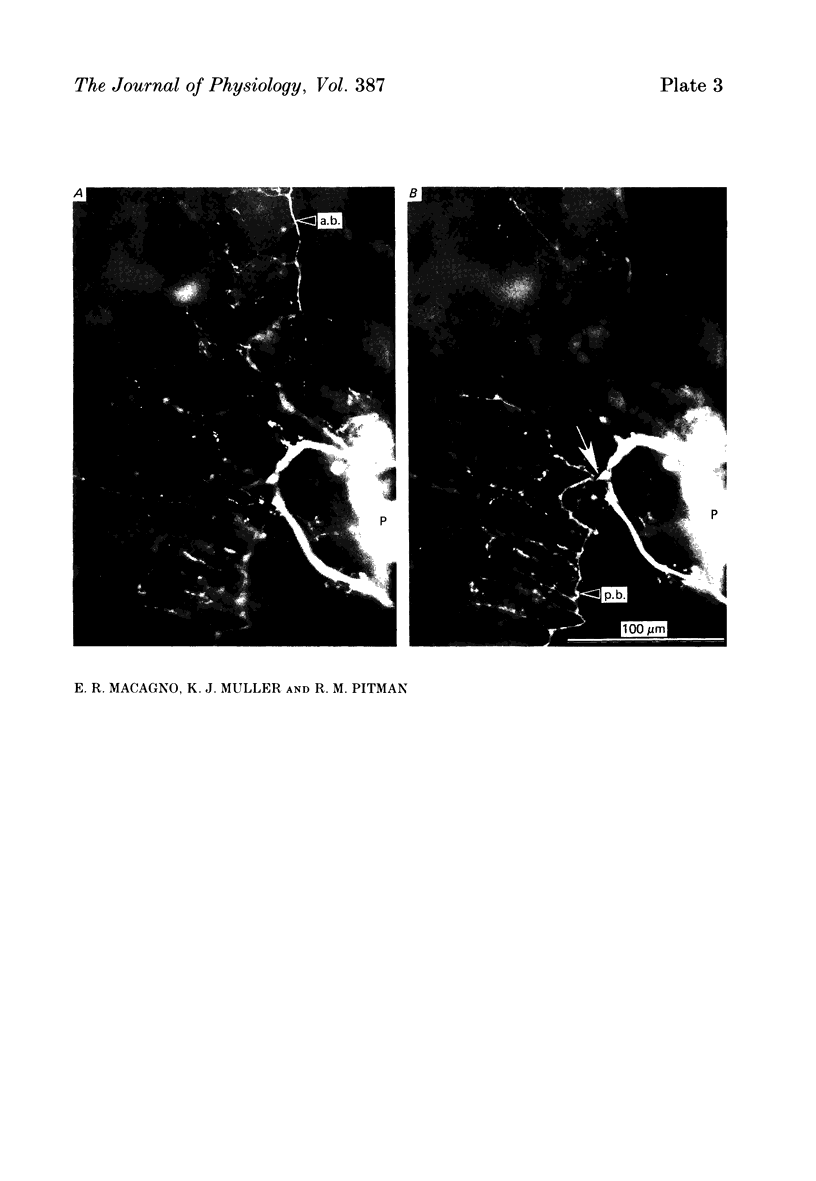
1. The pressure (P) sensory neurones innervating the ventral skin of the medicinal leech have receptive fields comprising a central region of skin innervated by two thicker axons and two neighbouring regions innervated by two thinner axons. Impulses originating in the thinner axons may fail to propagate through the central ganglion, apparently blocked at the branch point of large and small axons. 2. The P neurone excites the longitudinal (L) motoneurone, and blocked impulses originating in the anterior fine axon produce e.p.s.p.s that are less than one-half normal amplitude. Blocked impulses in the posterior fine axon are typically ineffective. 3. The branches of P and L neurones, marked with intracellularly injected horseradish peroxidase or with Lucifer Yellow, make synaptic contact at up to sixty-six sites within the neuropile. Of P neurone branches emerging from two fine axons, those from the posterior axon make fewer contacts, usually one or two at most, while branches from the anterior axon represent no more than half the total contacts. From cell to cell there is some variation in the total number of contacts, the distribution of branches, and the strength of transmission. 4. The locations of contacts measured morphologically correlate well with their distributions as predicted from reductions in e.p.s.p. amplitude during conduction block.
Full text
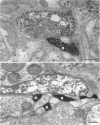
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. After-effects of nerve impulses on signalling in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):571–589. doi: 10.1113/jphysiol.1969.sp008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968 Jun;51(6):731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E. Morphology and distribution of touch cell terminals in the skin of the leech. J Physiol. 1981 Nov;320:219–228. doi: 10.1113/jphysiol.1981.sp013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E., Nicholls J. G., Parnas I. Physiological responses, receptive fields and terminal arborizations of nociceptive cells in the leech. J Physiol. 1982 May;326:251–260. doi: 10.1113/jphysiol.1982.sp014189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling D., Nicholls J., Parnas I. Destruction of a single cell in the central nervous system of the leech as a means of analysing its connexions and functional role. J Physiol. 1978 Sep;282:169–180. doi: 10.1113/jphysiol.1978.sp012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Raymond S. A., Lettvin J. Y. Multiple meaning in single visual units. Brain Behav Evol. 1970;3(1):72–101. doi: 10.1159/000125464. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRiemer S. A., Macagno E. R. Light microscopic analysis of contacts between pairs of identified leech neurons with combined use of horseradish peroxidase and lucifer yellow. J Neurosci. 1981 Jun;1(6):650–657. doi: 10.1523/JNEUROSCI.01-06-00650.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow B., Friesen W. O., Kristan W. B., Jr Physiological and morphological analysis of synaptic transmission between leech motor neurons. J Neurosci. 1985 Aug;5(8):2035–2050. doi: 10.1523/JNEUROSCI.05-08-02035.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Parnas I., Spira M. E. Differential conduction block in branches of a bifurcating axon. J Physiol. 1979 Oct;295:283–305. doi: 10.1113/jphysiol.1979.sp012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Spira M. E., Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973 Dec 21;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Jr, Muller K. J., Nicholls J. G. Persistent modification of synaptic interactions between sensory and motor nerve cells following discrete lesions in the central nervous system of the leech. J Physiol. 1974 Oct;242(2):289–305. doi: 10.1113/jphysiol.1974.sp010708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol. 1983 Jan;49(1):269–289. doi: 10.1152/jn.1983.49.1.269. [DOI] [PubMed] [Google Scholar]
- Lüscher H., Ruenzel P. W., Henneman E. Effects of impulse frequency, PTP, and temperature on responses elicited in large populations of motoneurons by impulses in single Ia-fibers. J Neurophysiol. 1983 Nov;50(5):1045–1058. doi: 10.1152/jn.1983.50.5.1045. [DOI] [PubMed] [Google Scholar]
- Macagno E. R., Levinthal C., Sobel I. Three-dimensional computer reconstruction of neurons and neuronal assemblies. Annu Rev Biophys Bioeng. 1979;8:323–351. doi: 10.1146/annurev.bb.08.060179.001543. [DOI] [PubMed] [Google Scholar]
- Macagno E. R., Muller K. J., Kristan W. B., Deriemer S. A., Stewart R., Granzow B. Mapping of neuronal contacts with intracellular injection of horseradish peroxidase and Lucifer yellow in combination. Brain Res. 1981 Jul 27;217(1):143–149. doi: 10.1016/0006-8993(81)90191-8. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., Duncan D. A quantitative study of morphological changes accompanying the initiation and progress of myelin production in the dorsal funiculus of the rat spinal cord. J Comp Neurol. 1971 May;142(1):1–22. doi: 10.1002/cne.901420102. [DOI] [PubMed] [Google Scholar]
- Muller K. J., McMahan U. J. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):481–499. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Muller K. J., Nicholls J. G. Different properties of synapses between a single sensory neurone and two different motor cells in the leech C.N.S. J Physiol. 1974 Apr;238(2):357–369. doi: 10.1113/jphysiol.1974.sp010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K. J., Scott S. A. Transmission at a 'direct' electrical connexion mediated by an interneurone in the leech. J Physiol. 1981 Feb;311:565–583. doi: 10.1113/jphysiol.1981.sp013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol. 1970 Aug;209(3):647–667. doi: 10.1113/jphysiol.1970.sp009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Bowling D. Killing of single neurons by intracellular injection of proteolytic enzymes. Nature. 1977 Dec 15;270(5638):626–628. doi: 10.1038/270626a0. [DOI] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972 Nov;35(6):903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Parnas I., Segev I. A mathematical model for conduction of action potentials along bifurcating axons. J Physiol. 1979 Oct;295:323–343. doi: 10.1113/jphysiol.1979.sp012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., McMahan U. J. The distribution of synapses on a physiologically identified motor neuron in the central nervous system of the leech. An electron microscope study after the injection of the fluorescent dye procion yellow. J Cell Biol. 1972 Oct;55(1):205–220. doi: 10.1083/jcb.55.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Nicholls J. Identified neurones isolated from leech CNS make selective connections in culture. Nature. 1979 Sep 6;281(5726):67–69. doi: 10.1038/281067a0. [DOI] [PubMed] [Google Scholar]
- Scott S. A., Muller K. J. Synapse regeneration and signals for directed axonal growth in the central nervous system of the leech. Dev Biol. 1980 Dec;80(2):345–363. doi: 10.1016/0012-1606(80)90410-8. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stuart A. E. Physiological and morphological properties of motoneurones in the central nervous system of the leech. J Physiol. 1970 Aug;209(3):627–646. doi: 10.1113/jphysiol.1970.sp009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol. 1973 May;230(3):509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W. Receptive fields, geometry and conduction block of sensory neurones in the central nervous system of the leech. J Physiol. 1976 Dec;263(3):513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]