Abstract
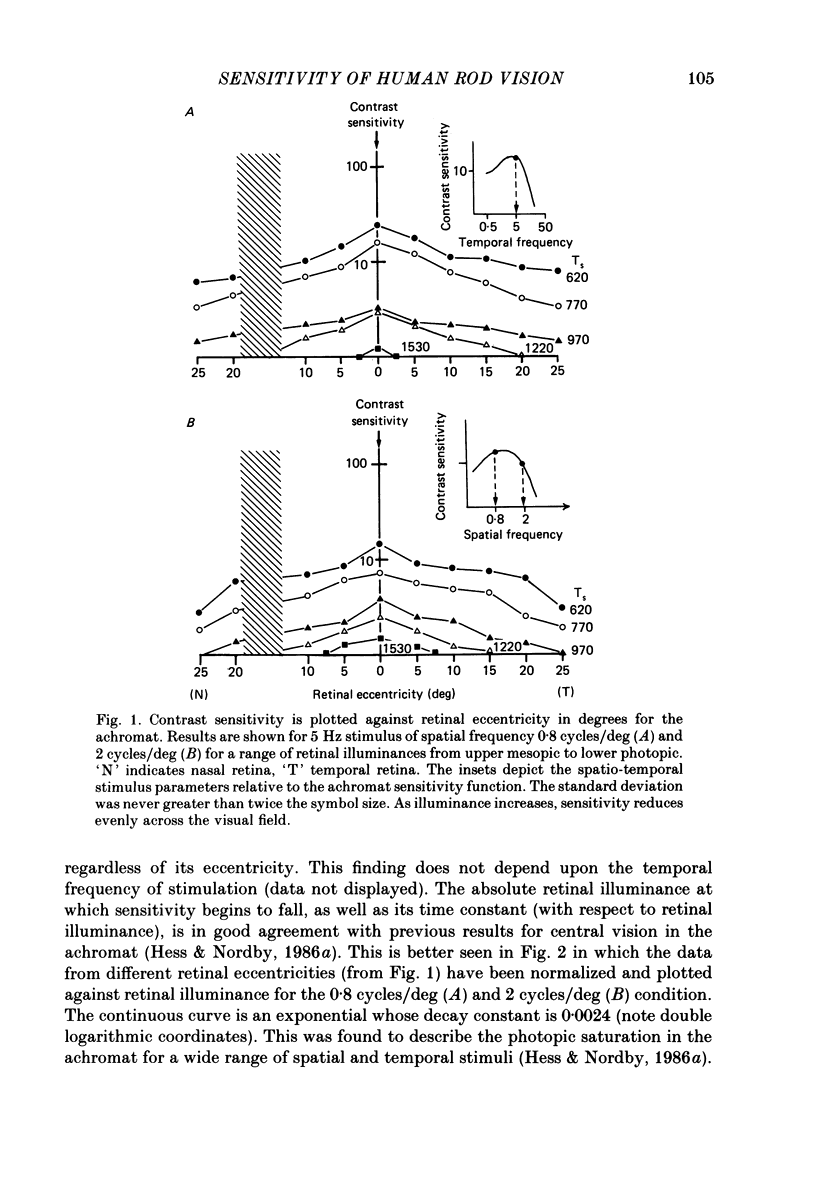
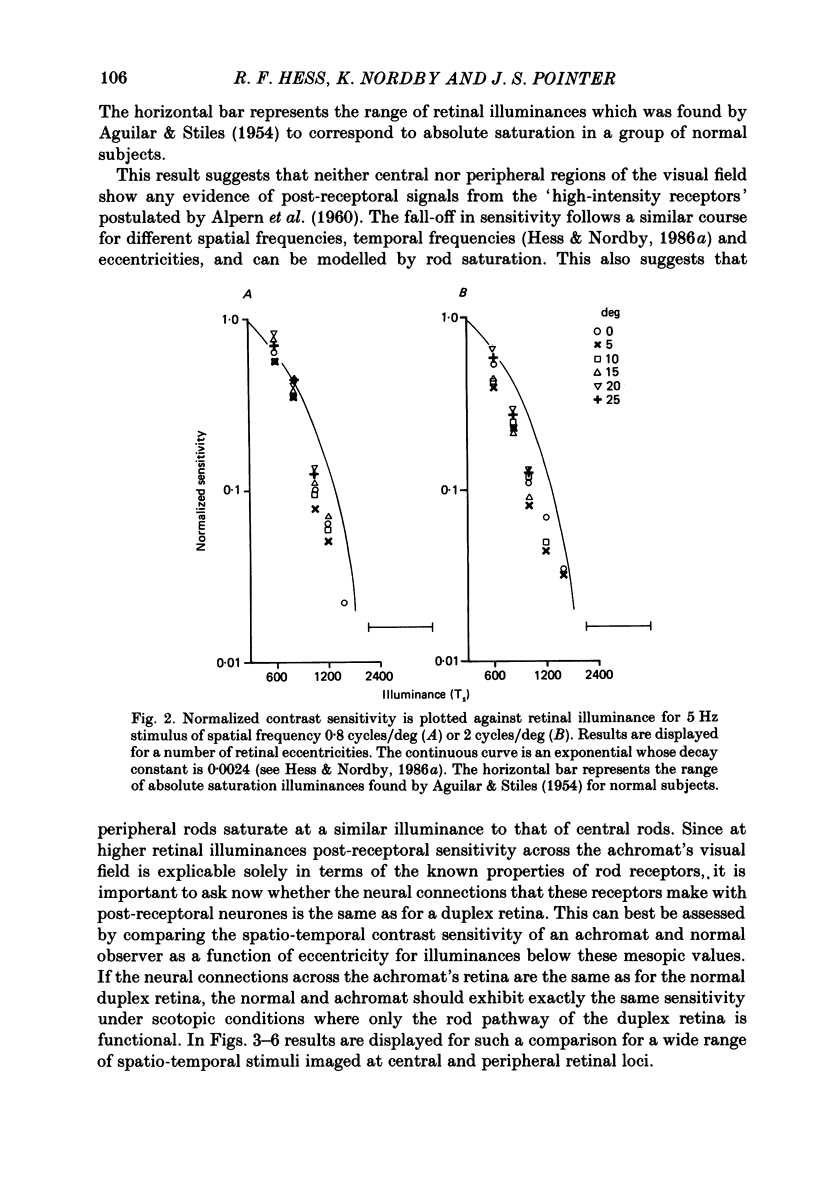
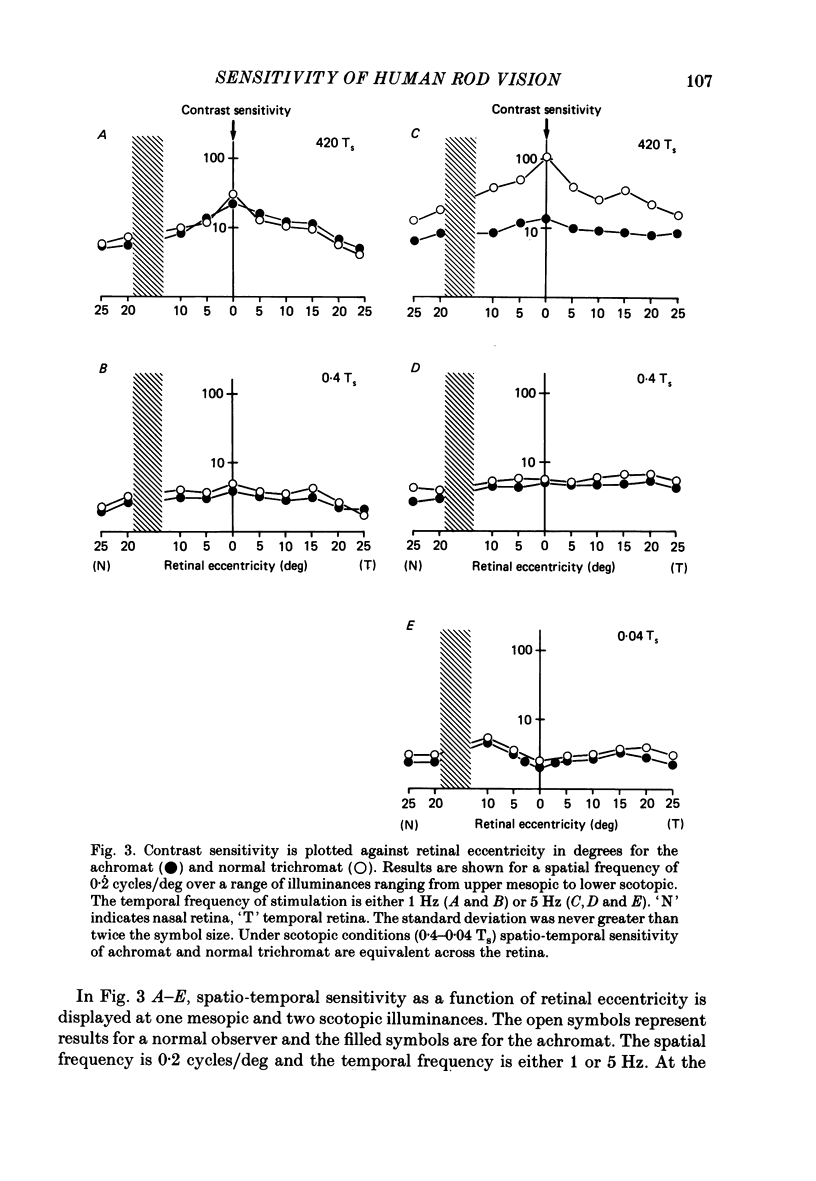
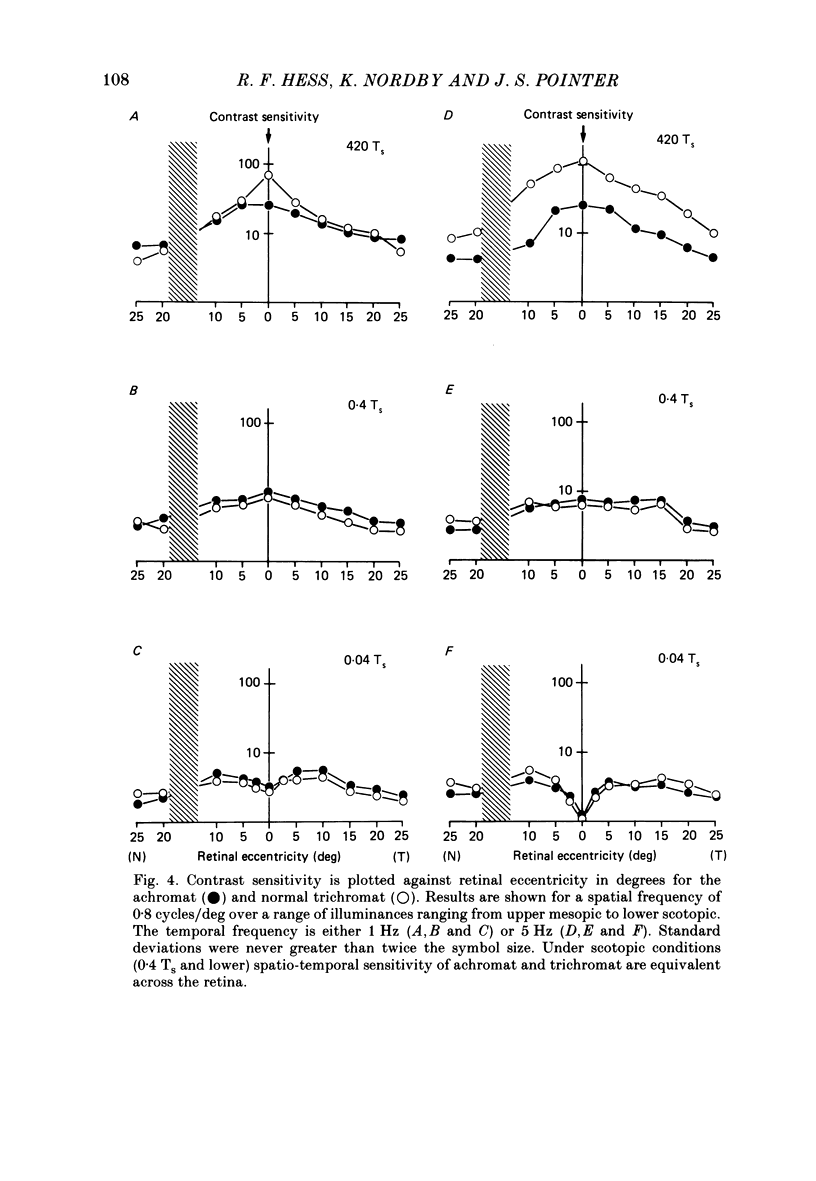
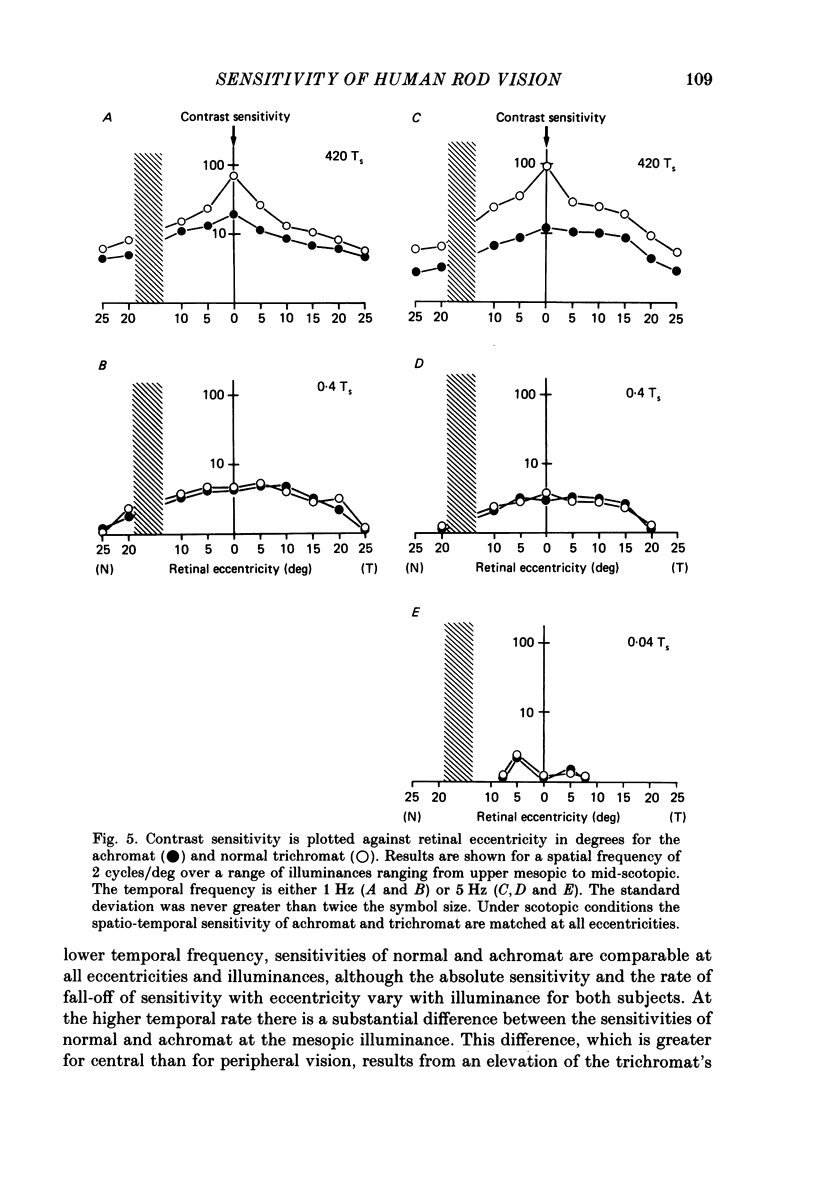
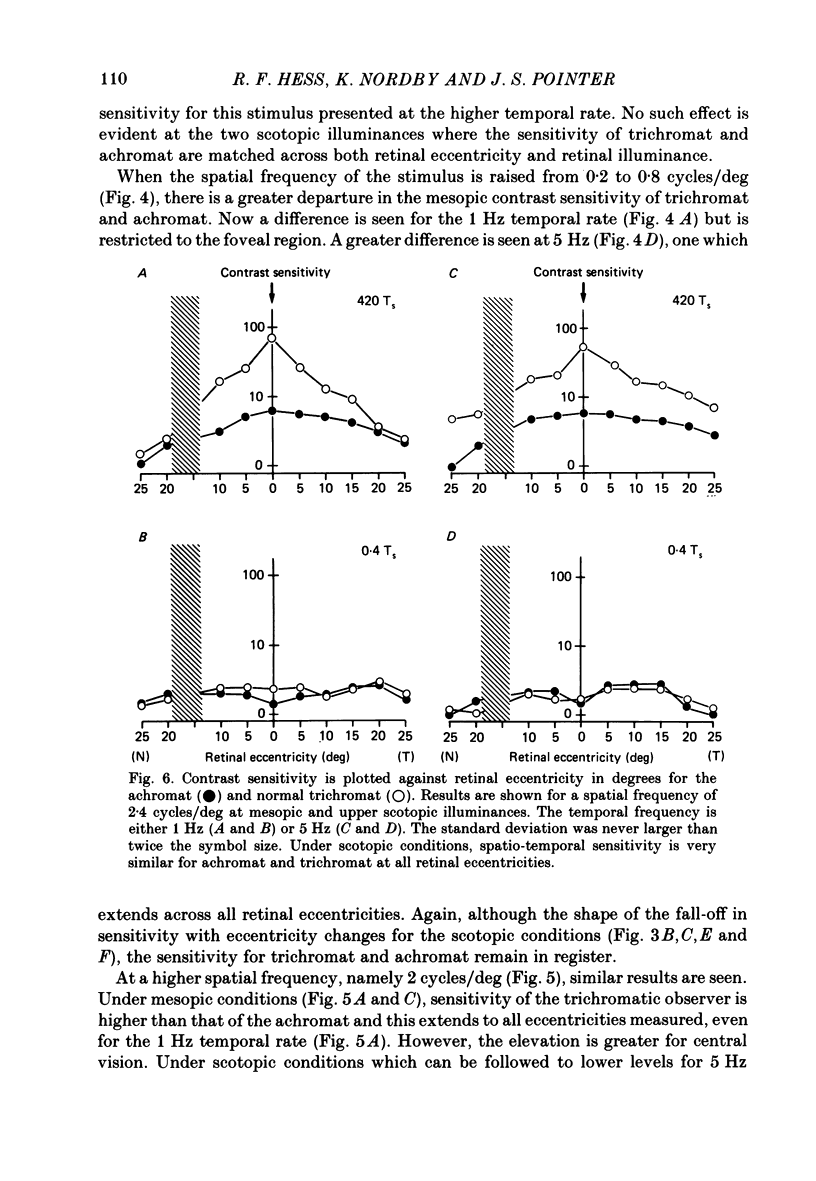
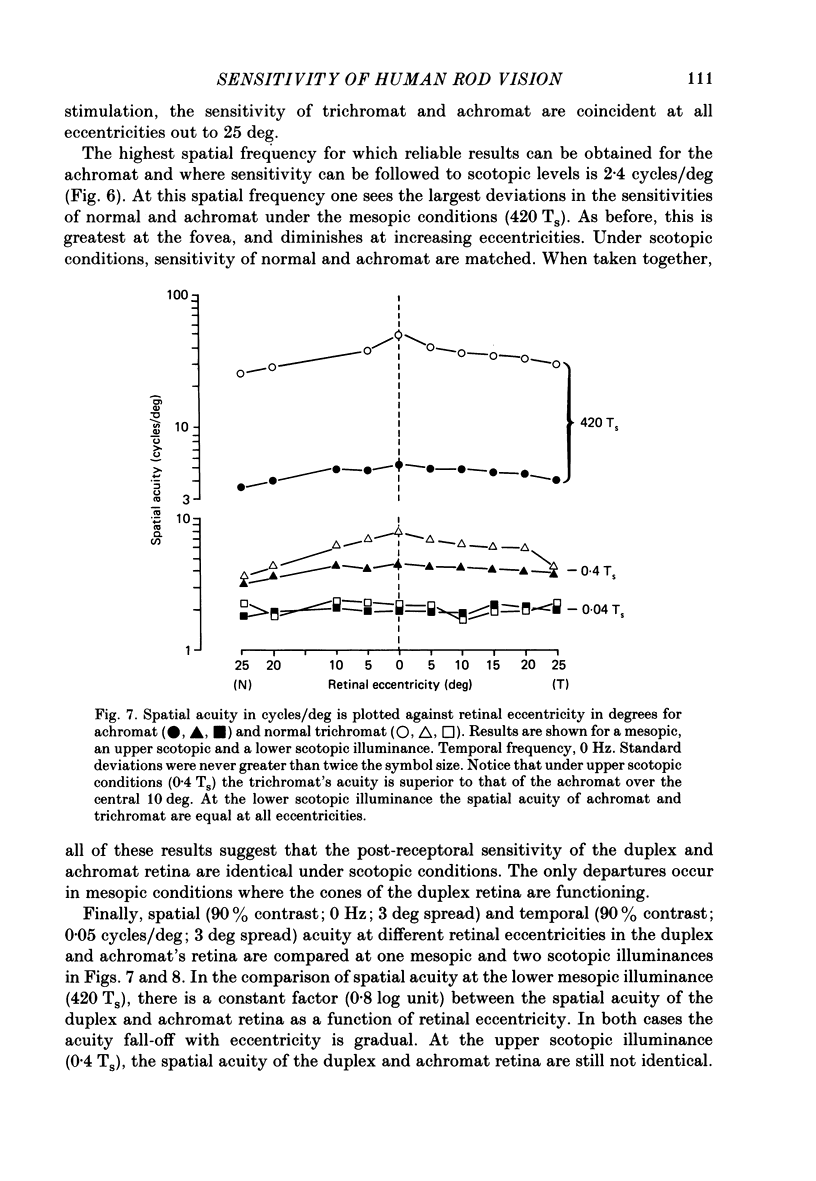
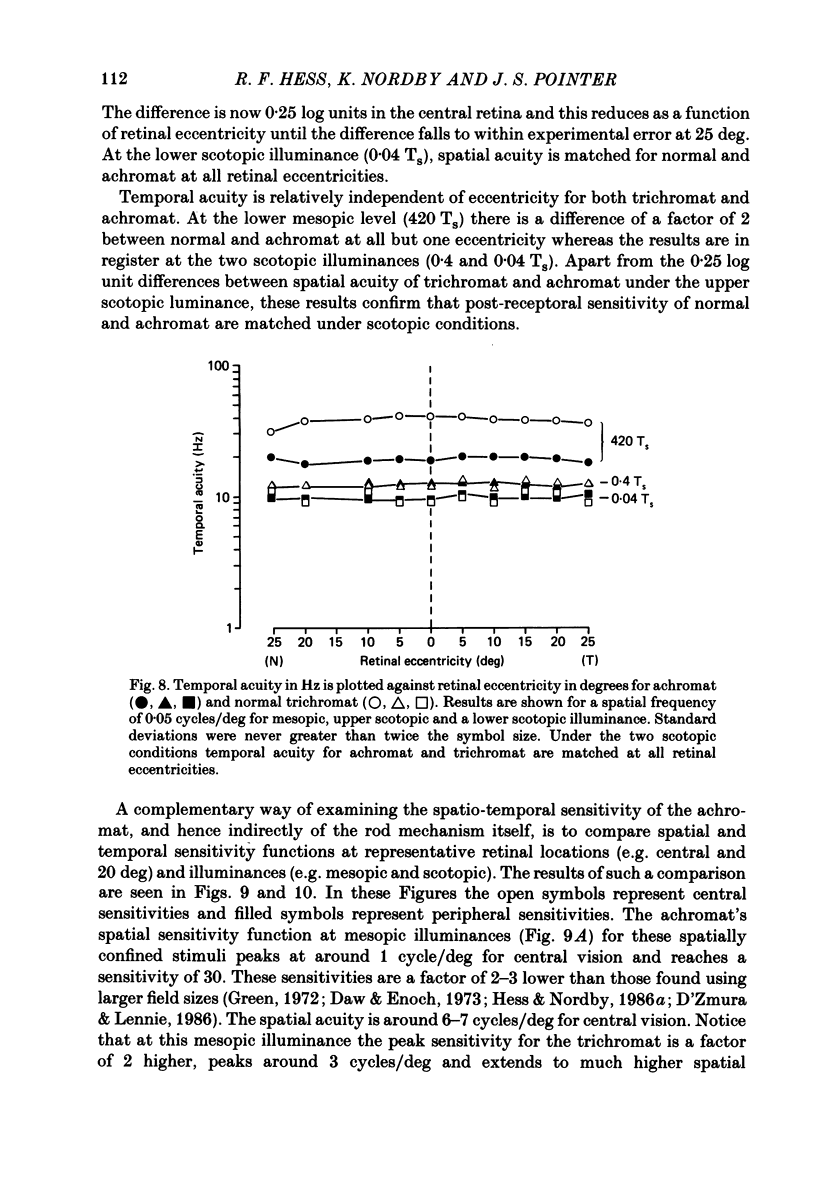
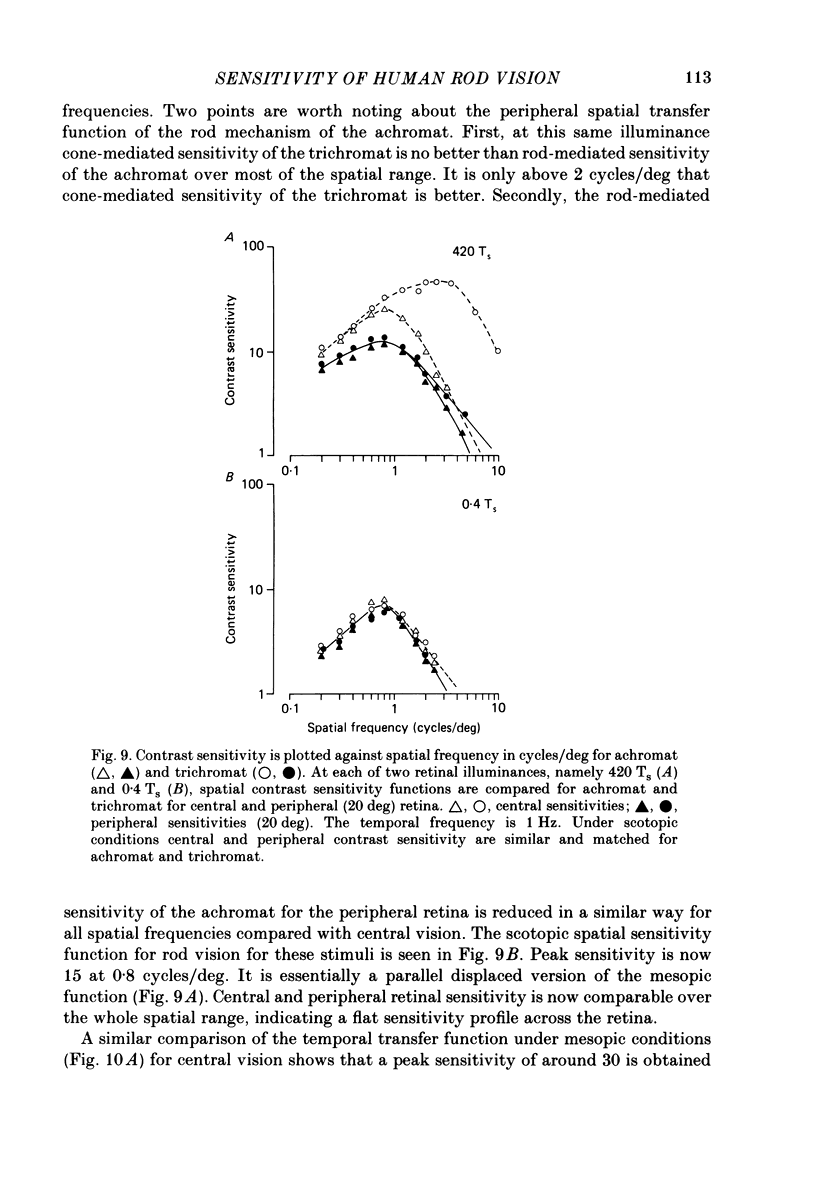
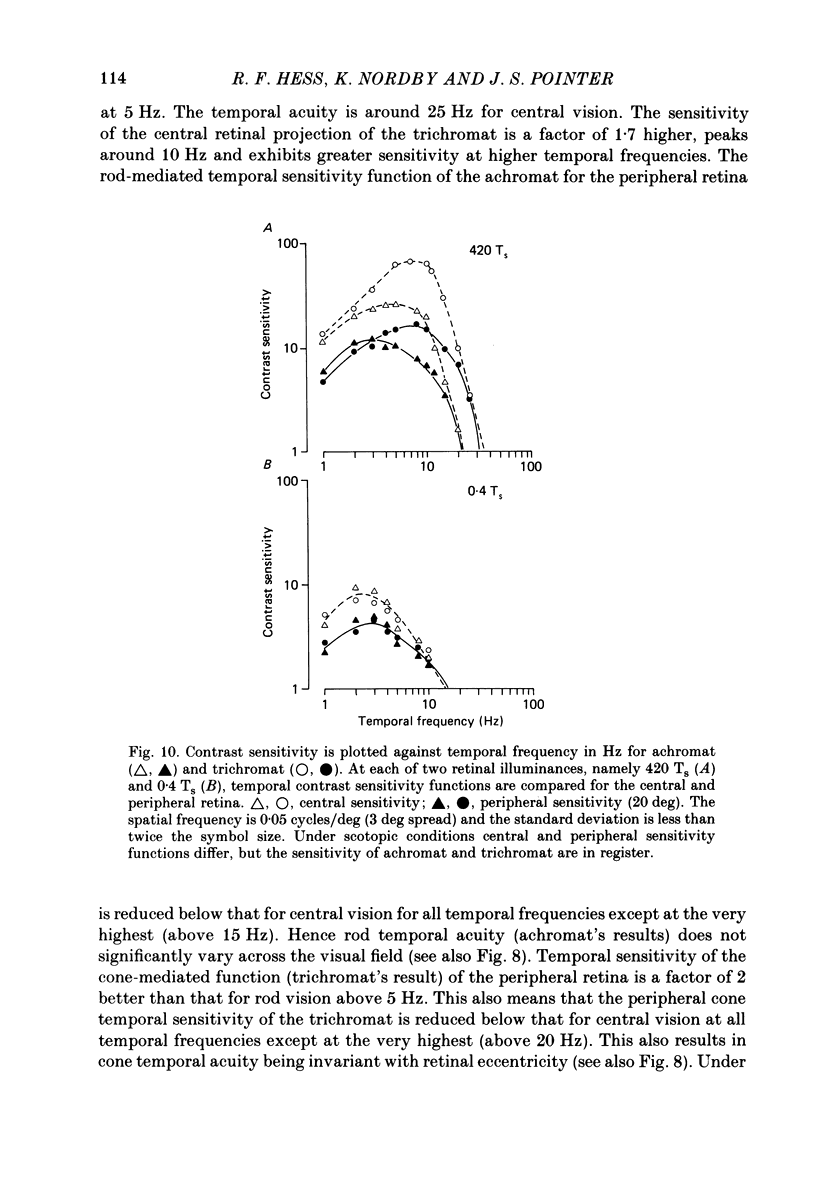
1. Detection thresholds for two-dimensional Gabor functions of varying spatial and temporal frequency were used to investigate the post-receptoral sensitivity across the retina of the typical and complete achromat. 2. Under photopic conditions there is no evidence for post-receptoral cone function at any retinal eccentricity investigated. Sensitivity saturates in a way consistent with known psychophysical and electrophysiological measures of rod saturation. This occurs in a unitary fashion across the retina. 3. Under scotopic conditions the regional fall-off in spatio-temporal sensitivity is similar for the achromat and duplex retina. This suggests that the rods in the achromat make normal neural connections. 4. Taken together this supports the contention that the typical and complete achromat is a functional rod monochromat and hence can be used to explore the sensitivity of the isolated rod post-receptoral mechanism under mesopic conditions where its sensitivity is optimal. This is where its contribution is most difficult to isolate in the duplex retina. 5. For the human rod mechanism, mesopic post-receptoral sensitivity for all spatio-temporal stimuli is optimal in the central region of the retina and falls off as a function of eccentricity. 6. For localized stimuli, peripheral spatial sensitivity is reduced evenly at all spatial frequencies compared with that of the central retina. A similar displacement of the spatial sensitivity function of the rod mechanism occurs as illuminance is reduced. 7. For localized stimuli, temporal acuity of the rod mechanism is around 20-25 Hz irrespective of retinal position. As the illuminance is further lowered dynamics of the rod pathway are reduced irrespective of retinal position and the sensitivity function maintains a bandpass shape. 8. The regional distribution sensitivity of the rod mechanism changes as illuminance is reduced from mesopic to scotopic levels.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERN M., FALLS H. F., LEE G. B. The enigma of typical total monochromacy. Am J Ophthalmol. 1960 Nov;50:996–1012. doi: 10.1016/0002-9394(60)90353-6. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. B., Rushton W. A. Dark adaptation and increment threshold in a rod monochromat. J Physiol. 1965 Dec;181(3):612–628. doi: 10.1113/jphysiol.1965.sp007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner J. D. The temporal properties of rod vision. J Physiol. 1982 Nov;332:139–155. doi: 10.1113/jphysiol.1982.sp014406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Zmura M., Lennie P. Shared pathways for rod and cone vision. Vision Res. 1986;26(8):1273–1280. doi: 10.1016/0042-6989(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Daitch J. M., Green D. G. Contrast sensitivity of the human peripheral retina. Vision Res. 1969 Aug;9(8):947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Enoch J. M. Contrast sensitivity, Westheimer function and Stiles-Crawford effect in a blue cone monochromat. Vision Res. 1973 Sep;13(9):1669–1680. doi: 10.1016/0042-6989(73)90086-2. [DOI] [PubMed] [Google Scholar]
- Falls H. F., Wolter J. R., Alpern M. Typical total monochromacy. A histological and psychophysical study. Arch Ophthalmol. 1965 Nov;74(5):610–616. doi: 10.1001/archopht.1965.00970040612005. [DOI] [PubMed] [Google Scholar]
- Glickstein M., Heath G. G. Receptors in the monochromat eye. Vision Res. 1975 Jun;15(6):633–636. doi: 10.1016/0042-6989(75)90276-x. [DOI] [PubMed] [Google Scholar]
- Green D. G. Visual acuity in the blue cone monochromat. J Physiol. 1972 Apr;222(2):419–426. doi: 10.1113/jphysiol.1972.sp009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON R., HOEFNAGEL D., HAYWARD J. N. Congenital total color blindness: a clincopathological report. Arch Ophthalmol. 1960 Nov;64:685–692. doi: 10.1001/archopht.1960.01840010687010. [DOI] [PubMed] [Google Scholar]
- Hess R. F., Nordby K. Spatial and temporal limits of vision in the achromat. J Physiol. 1986 Feb;371:365–385. doi: 10.1113/jphysiol.1986.sp015981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., Nordby K. Spatial and temporal properties of human rod vision in the achromat. J Physiol. 1986 Feb;371:387–406. doi: 10.1113/jphysiol.1986.sp015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971 Feb;49(2 Suppl):467+–467+. [PubMed] [Google Scholar]
- Nordby K., Stabell B., Stabell U. Dark-adaptation of the human rod system. Vision Res. 1984;24(8):841–849. doi: 10.1016/0042-6989(84)90156-1. [DOI] [PubMed] [Google Scholar]
- Nunn B. J., Baylor D. A. Visual transduction in retinal rods of the monkey Macaca fascicularis. Nature. 1982 Oct 21;299(5885):726–728. doi: 10.1038/299726a0. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Cowey A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience. 1984 Aug;12(4):1125–1137. doi: 10.1016/0306-4522(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Robson J. G., Graham N. Probability summation and regional variation in contrast sensitivity across the visual field. Vision Res. 1981;21(3):409–418. doi: 10.1016/0042-6989(81)90169-3. [DOI] [PubMed] [Google Scholar]
- SLOAN L. L. Congenital achromatopsia; a report of 19 cases. J Opt Soc Am. 1954 Feb;44(2):117–128. doi: 10.1364/josa.44.000117. [DOI] [PubMed] [Google Scholar]
- Sakitt B. Psychophysical correlates of photoreceptor activity. Vision Res. 1976;16(2):129–140. doi: 10.1016/0042-6989(76)90089-4. [DOI] [PubMed] [Google Scholar]
- Volkmann F. C., Riggs L. A., White K. D., Moore R. K. Contrast sensitivity during saccadic eye movements. Vision Res. 1978;18(9):1193–1199. doi: 10.1016/0042-6989(78)90104-9. [DOI] [PubMed] [Google Scholar]
- WALLS G. L., HEATH G. G. Typical total color blindness reinterpreted. Acta Ophthalmol (Copenh) 1954;32(3):253–contd. [PubMed] [Google Scholar]


