Abstract
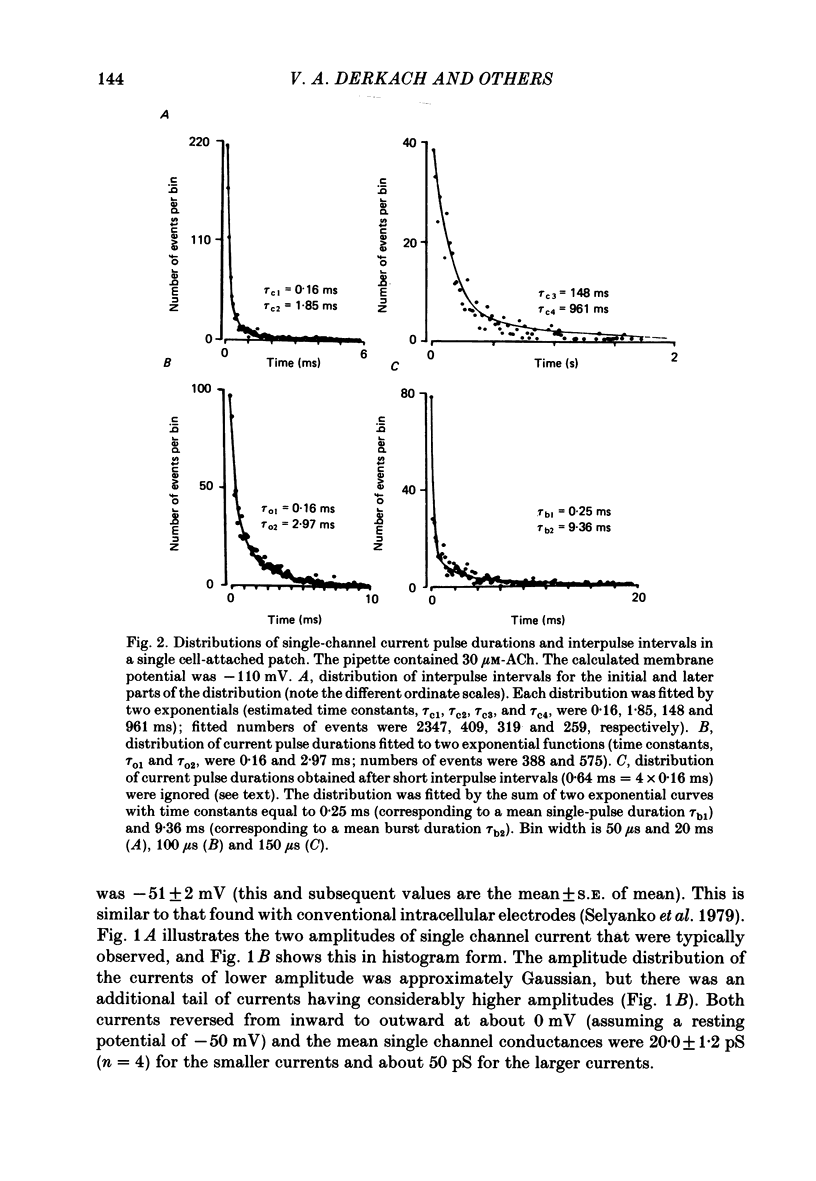
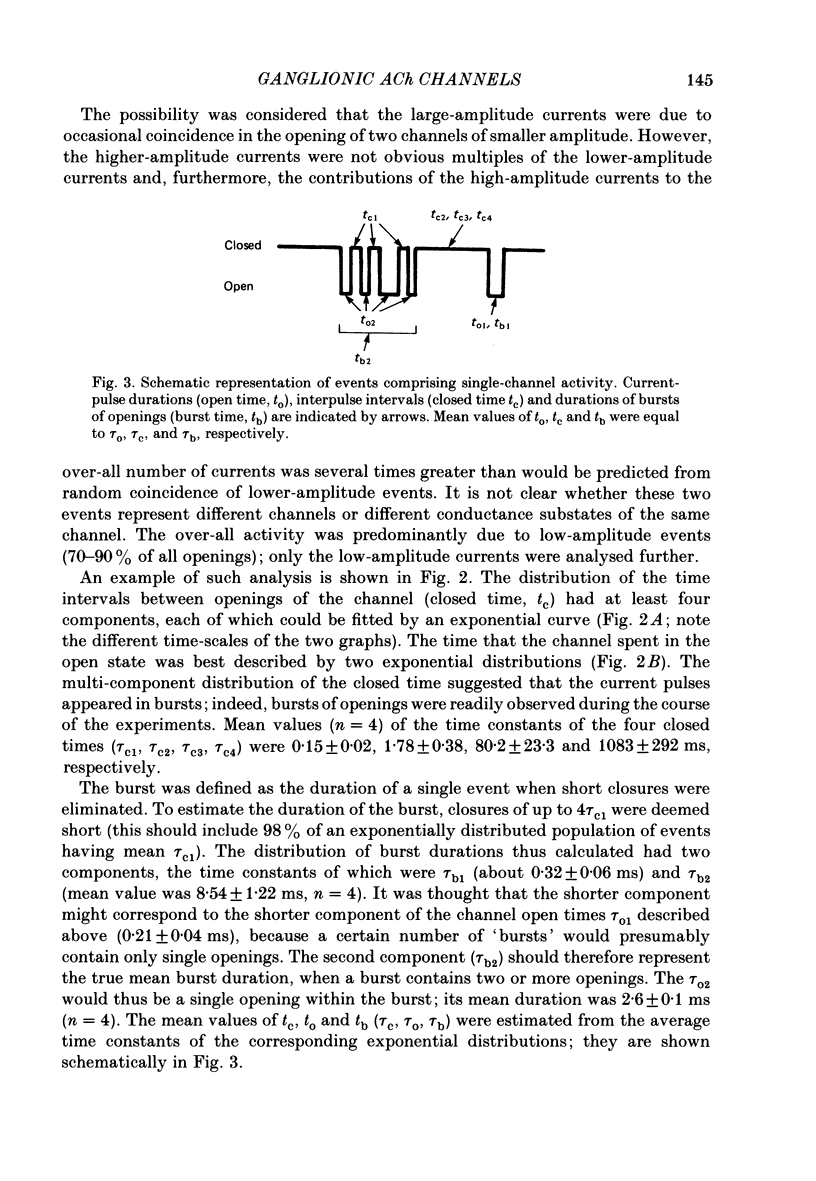
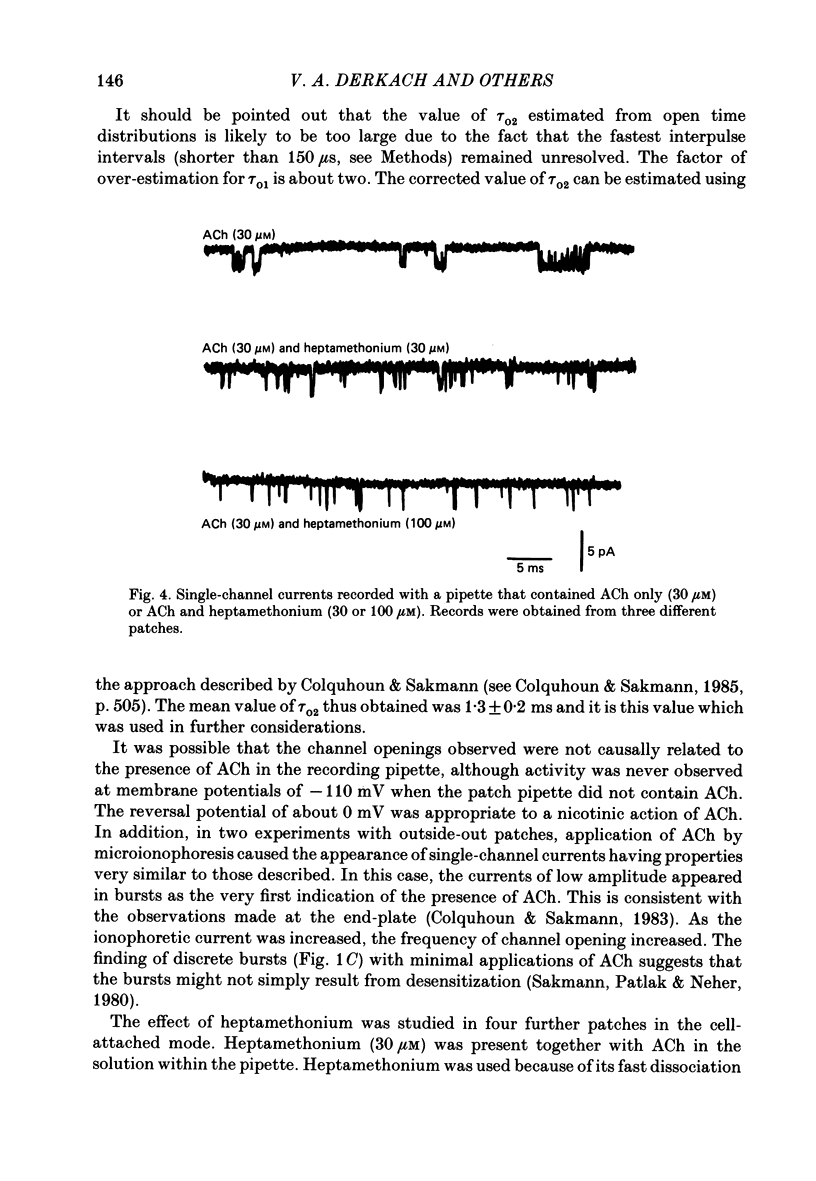
1. The elementary currents flowing through single channels opened by acetylcholine were recorded in rat superior cervical ganglion neurones using patch-clamp methods. Acetylcholine (30 microM) was included in the patch electrode (cell-attached recordings) or applied by ionophoresis (outside-out configuration). All measurements were made at 23-25 degrees C and mostly at -110 mV. 2. Channel openings appeared both as single events and as bursts of events. One population of the currents observed had a conductance of 20.0 +/- 0.2 pS (mean +/- S.E. of mean, n = 4). A second population had a conductance of about 50 pS, occurred more rarely, and was not included in further analysis. 3. Four channel closed time periods and two channel open time periods were found from the distributions of closed and open times. It was found that shorter channel openings (about 0.2 ms) appeared in isolation, whereas longer openings (duration 1.3 +/- 0.2 ms, n = 4) appeared as bursts of openings separated by the shortest channel closed time periods (about 0.15 ms). The next shortest closed time (about 2 ms) apparently corresponds to the lifetime of the channel not activated by acetylcholine. The two longer closed times (about 80 ms and 1 s) may reflect desensitization. The mean burst duration was 8.5 +/- 1.2 ms (n = 4), giving about six openings per burst. 4. Because the time constant of decay of the excitatory post-synaptic current is more similar to the burst duration than to the duration of individual single openings, it is suggested that acetylcholine released from presynaptic nerves may result in a burst of openings rather than a single opening. 5. On the basis of the above assumption, the rate constants were calculated for a sequential model in which acetylcholine binds to the receptor (forward rate k + 1 = 2.3 X 10(7) M-1 s-1; reverse rate k-1 = 1235 s-1) which then undergoes a conformational change to an open state (forward rate beta = 6293 s-1; reverse rate alpha = 894 s-1). 6. When heptamethonium (30 microM) was added to the solution in the patch electrode, the burst duration was markedly shortened, but there was no change in the closed time between two openings within the burst. This effect was voltage-dependent, which suggests that heptamethonium binds to the channel after it is opened by acetylcholine.
Full text
PDF

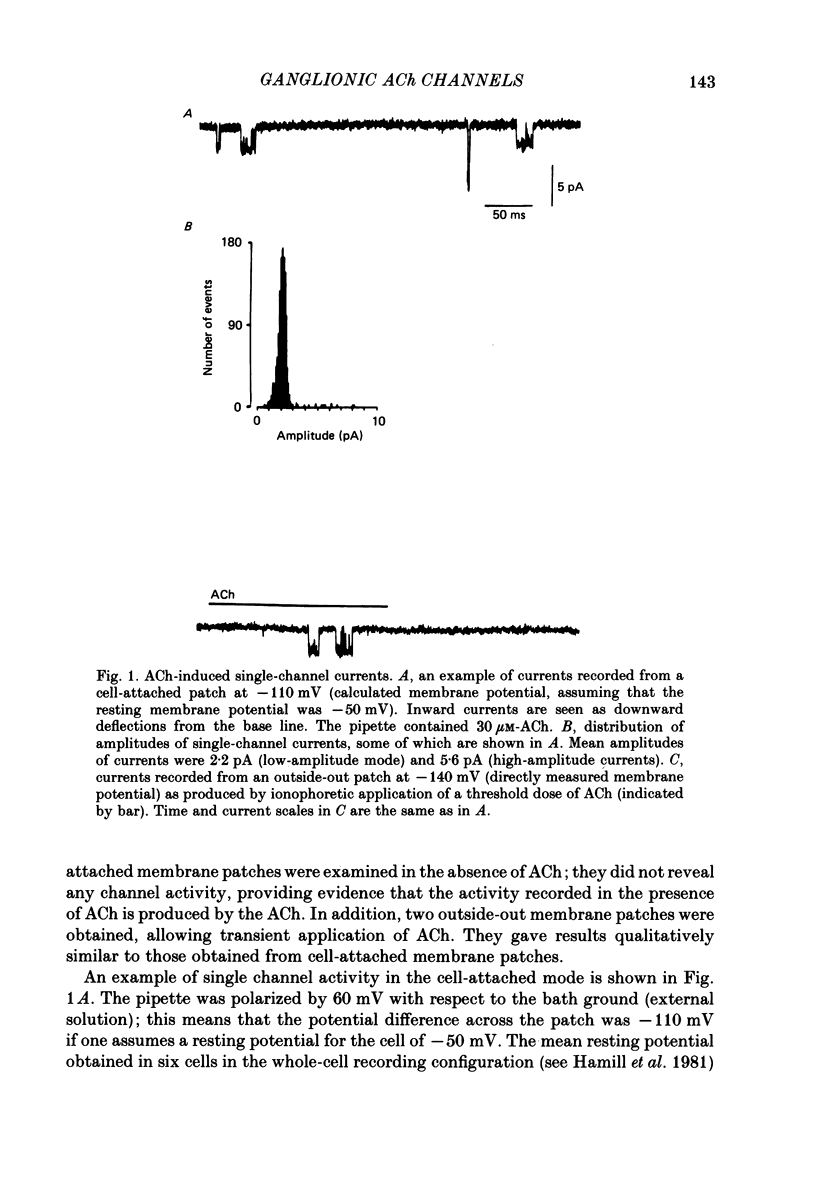





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Derkach V. A., Selyanko A. A., Skok V. I. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983 Mar;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris A. J., Kuffler S. W., Dennis M. J. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):541–553. doi: 10.1098/rspb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Head S. D. Temperature and end-plate currents in rat diaphragm. J Physiol. 1983 Jan;334:441–459. doi: 10.1113/jphysiol.1983.sp014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McLachlan E. M. Post-natal development of ganglia in the lower lumbar sympathetic chain of the rat. J Physiol. 1984 Apr;349:119–134. doi: 10.1113/jphysiol.1984.sp015147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Gray P. T., Colquhoun D., Rang H. P. Kinetics of acetylcholine activated ion channels in chick ciliary ganglion neurones grown in tissue culture. Pflugers Arch. 1984 Jan;400(1):44–50. doi: 10.1007/BF00670535. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A., Derkach V. A., Skok V. I. Fast excitatory postsynaptic currents in voltage-clamped mammalian sympathetic ganglion neurones. J Auton Nerv Syst. 1979 Dec;1(2):127–137. doi: 10.1016/0165-1838(79)90011-0. [DOI] [PubMed] [Google Scholar]
- Skok V. I. Channel-blocking mechanism ensures specific blockade of synaptic transmission. Neuroscience. 1986;17(1):1–9. doi: 10.1016/0306-4522(86)90221-6. [DOI] [PubMed] [Google Scholar]


