Abstract
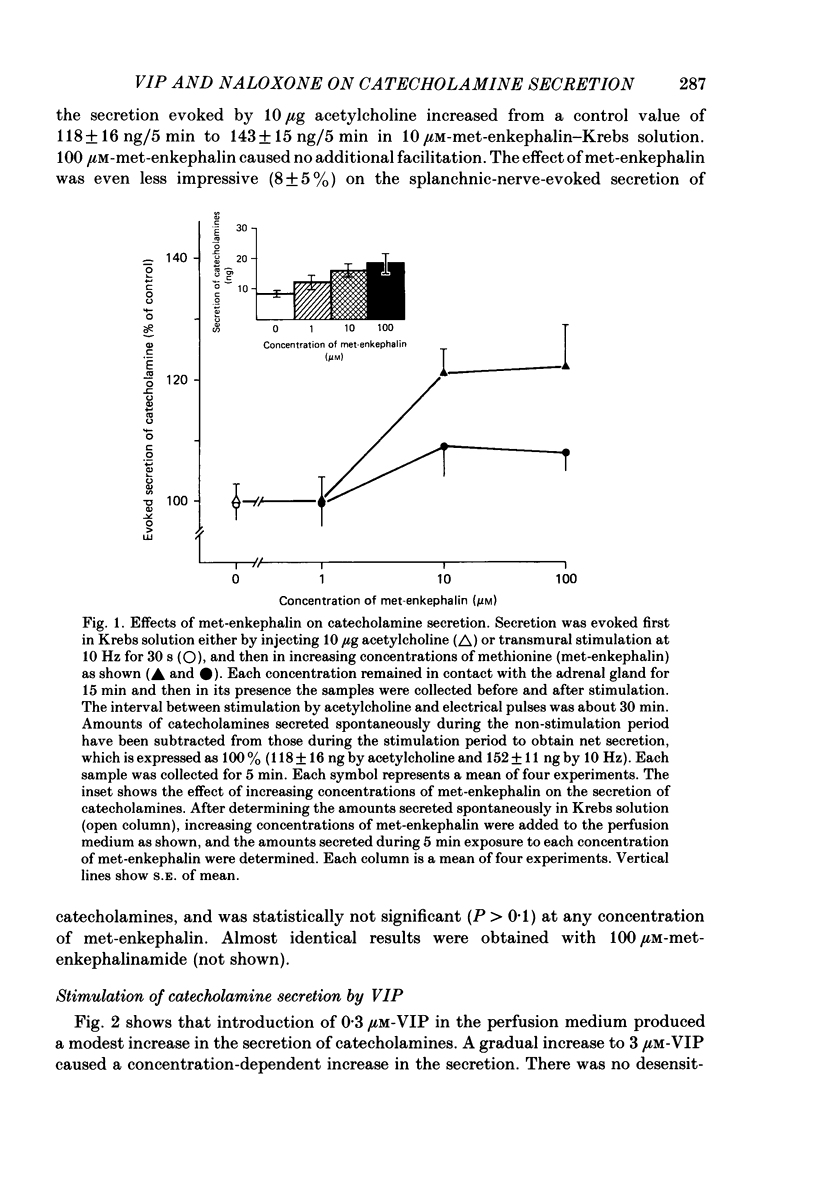
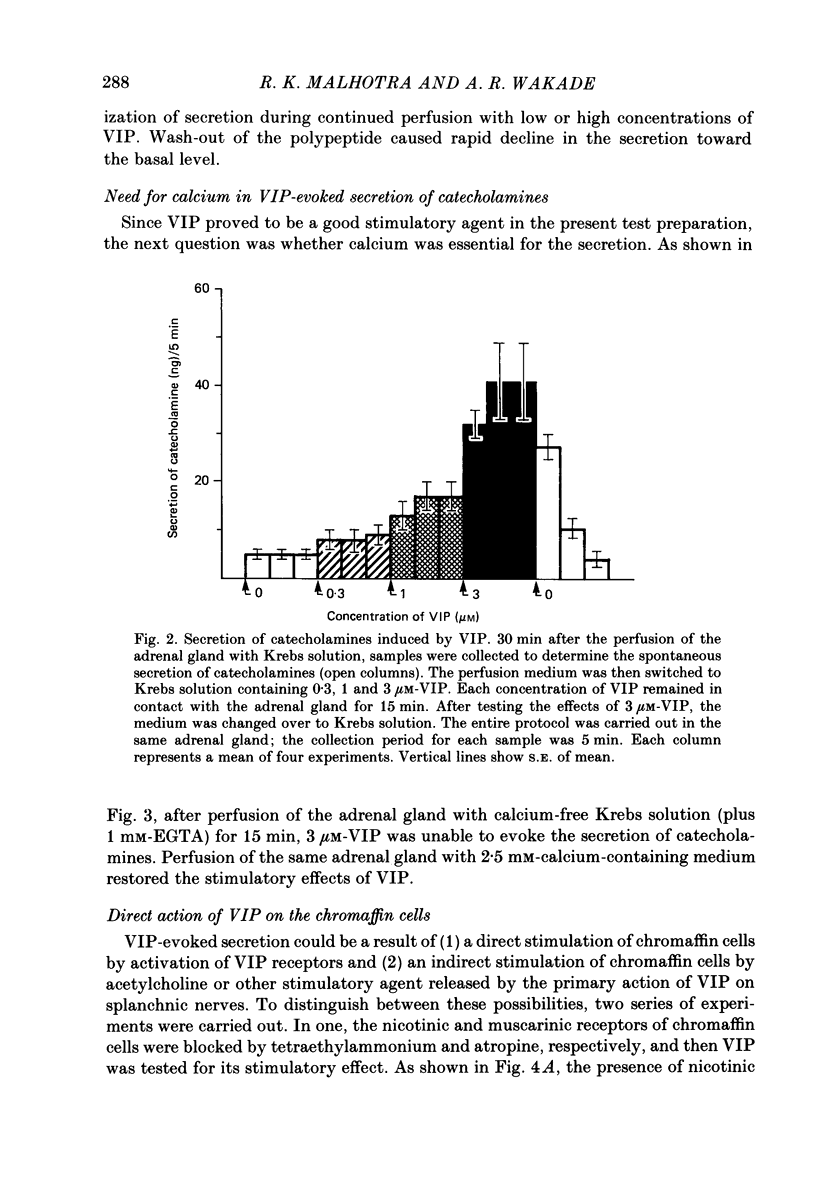
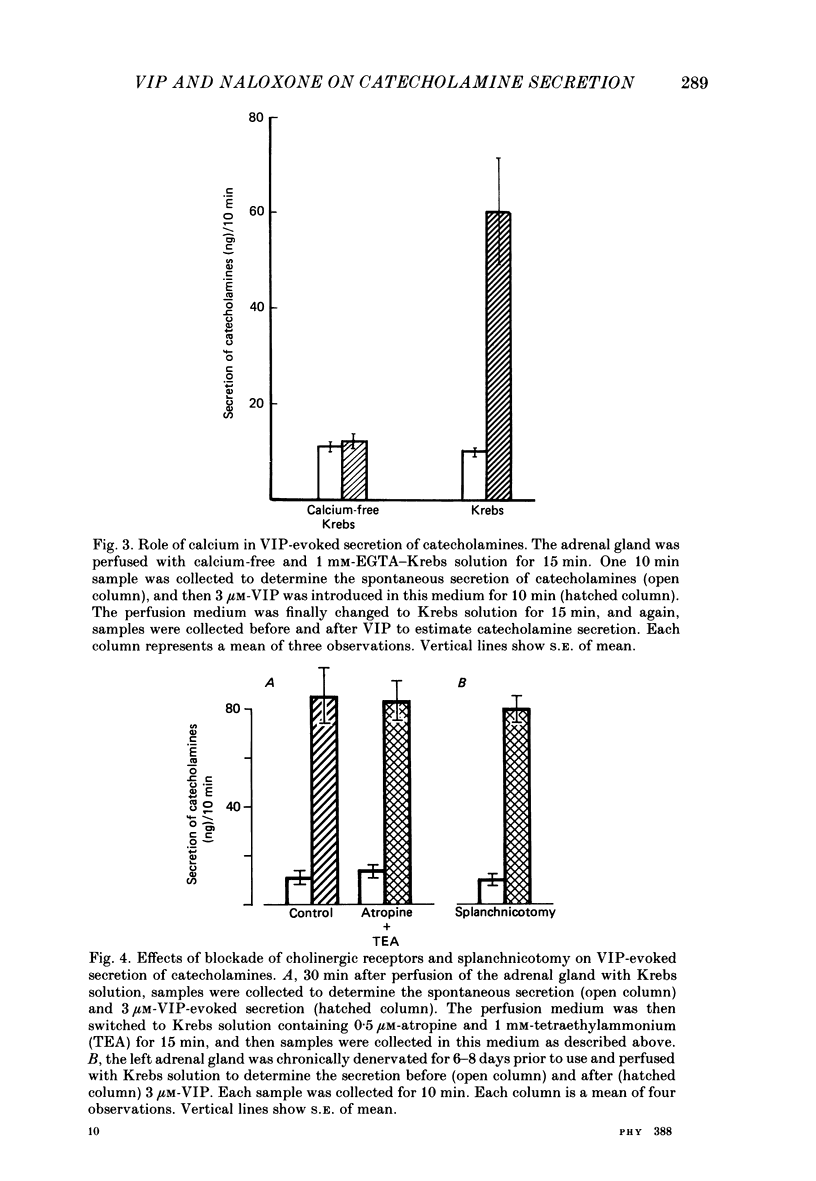
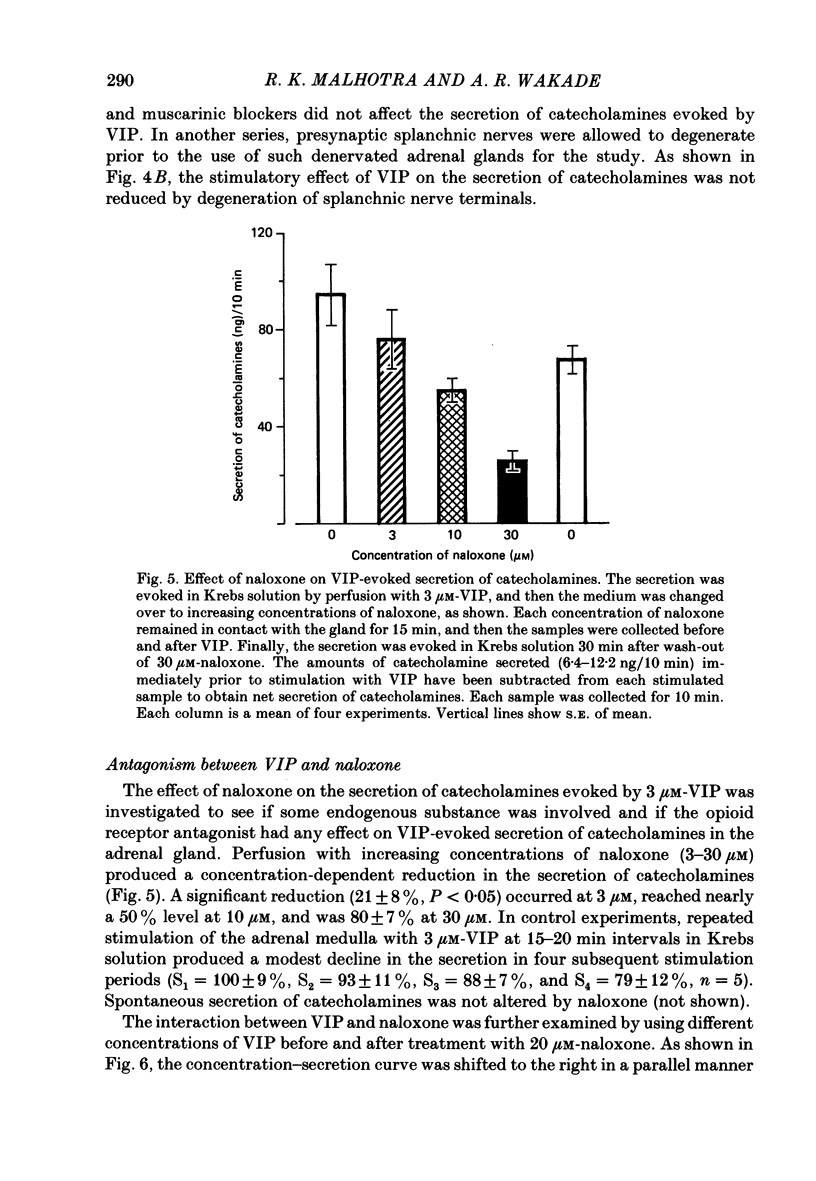
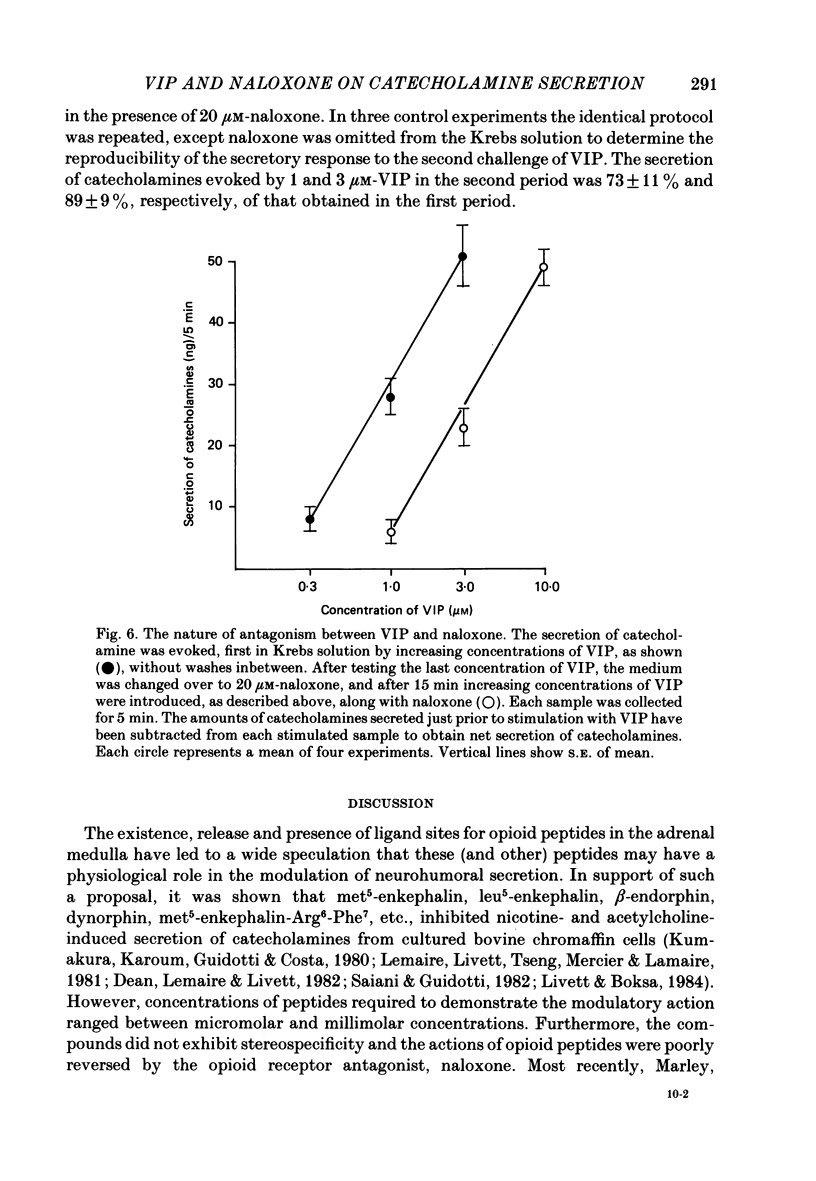
1. Our previous studies have indicated that splanchnic nerves release a substance(s), other than acetylcholine, that induces the secretion of catecholamines from the rat adrenal medulla. To identify the nature of the non-cholinergic substance, the effects of met-enkephalin and vasoactive intestinal polypeptide (VIP) were investigated in the perfused adrenal gland of the rat. 2. The secretion of catecholamines increased from a basal level of 8 ng to a maximum value of 18 ng during perfusion with 100 microM-met-enkephalin. The secretion evoked by 10 micrograms acetylcholine increased from 118 to 143 ng in the presence of 10 microM-met-enkephalin. Higher concentrations of met-enkephalin (100 microM) had no additional effect. Secretion of catecholamines evoked by stimulation of splanchnic nerves (10 Hz for 30 s) was even less (8%) affected by met-enkephalin. 3. 0.3 microM-VIP caused a significant increase in the secretion of catecholamines, and the effect increased with an increase in the concentration of VIP. About 115 ng of catecholamines were secreted during 15 min perfusion with 3 microM-VIP. 4. VIP-evoked secretion was not affected by antagonists of nicotinic and muscarinic receptors, nor by chronic splanchnicotomy. However, removal of calcium ions from, and inclusion of 1 mM-EGTA in, the perfusion medium completely inhibited the secretion evoked by VIP. 5. VIP-evoked secretion was reduced (20-75%) in a concentration-dependent manner by 3-30 microM-naloxone. 6. It is suggested that VIP may be the non-cholinergic excitatory substance present in the splanchnic nerves and released along with acetylcholine during simulation of the nerves to evoke secretion of catecholamine from the rat chromaffin cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Dean D. M., Lemaire S., Livett B. G. Evidence that inhibition of nicotine-mediated catecholamine secretion from adrenal chromaffin cells by enkephalin, beta-endorphin, dynorphin (1-13), and opiates is not mediated via specific opiate receptors. J Neurochem. 1982 Mar;38(3):606–614. doi: 10.1111/j.1471-4159.1982.tb08674.x. [DOI] [PubMed] [Google Scholar]
- Di Giulio A. M., Yang H. Y., Fratta W., Costa E. Decreased content of immunoreactive enkephalin-like peptide in peripheral tissues of spontaneously hypertensive rats. Nature. 1979 Apr 12;278(5705):646–647. doi: 10.1038/278646a0. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Iversen L. L., Breuker E. Naloxone as a GABA antagonist: evidence from iontophoretic, receptor binding and convulsant studies. Eur J Pharmacol. 1978 Jan 1;47(1):19–27. doi: 10.1016/0014-2999(78)90369-2. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J. Vasoactive intestinal polypeptide: measurement, distribution and putative neurotransmitter function. Digestion. 1979;19(3):149–169. doi: 10.1159/000198339. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Said S. I., Reynolds R. C., Koniges F. C. Vasoactive intestinal polypeptide in brain: localization in and release from isolated nerve terminals. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3424–3428. doi: 10.1073/pnas.74.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Fahrenkrug J. Immunohistochemical evidence for a local VIP-ergic neuron system in the adrenal gland of the rat. Acta Physiol Scand. 1981 Dec;113(4):575–576. doi: 10.1111/j.1748-1716.1981.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Ho C. K., Zigmond R. E. Secretin and vasoactive intestinal peptide acutely increase tyrosine 3-monooxygenase in the rat superior cervical ganglion. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7566–7569. doi: 10.1073/pnas.79.23.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kyoshima K., Olschowka J. A., Jacobowitz D. M. Vasoactive intestinal polypeptide immunoreactive and cholinergic nerves in the whole mount preparation of the major cerebral arteries of the rat. Histochemistry. 1983;79(3):377–381. doi: 10.1007/BF00491773. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- Lemaire S., Livett B., Tseng R., Mercier P., Lemaire I. Studies on the inhibitory action of opiate compounds in isolated bovine adrenal chromaffin cells: noninvolvement of stereospecific opiate binding sites. J Neurochem. 1981 Mar;36(3):886–892. doi: 10.1111/j.1471-4159.1981.tb01677.x. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Boksa P. Receptors and receptor modulation in cultured chromaffin cells. Can J Physiol Pharmacol. 1984 Apr;62(4):467–476. doi: 10.1139/y84-076. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Emson P., Fahrenkrug J., Hökfelt T. Vasoactive intestinal polypeptide and cholinergic mechanisms in cat nasal mucosa: studies on choline acetyltransferase and release of vasoactive intestinal polypeptide. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5255–5259. doi: 10.1073/pnas.78.8.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade A. R. Non-cholinergic component of rat splanchnic nerves predominates at low neuronal activity and is eliminated by naloxone. J Physiol. 1987 Feb;383:639–652. doi: 10.1113/jphysiol.1987.sp016434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley P. D., Mitchelhill K. I., Livett B. G. Effects of opioid peptides containing the sequence of Met5-enkephalin or Leu5-enkephalin on nicotine-induced secretion from bovine adrenal chromaffin cells. J Neurochem. 1986 Jan;46(1):1–11. doi: 10.1111/j.1471-4159.1986.tb12918.x. [DOI] [PubMed] [Google Scholar]
- Saiani L., Guidotti A. Opiate receptor-mediated inhibition of catecholamine release in primary cultures of bovine adrenal chromaffin cells. J Neurochem. 1982 Dec;39(6):1669–1676. doi: 10.1111/j.1471-4159.1982.tb08001.x. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Facilitation of secretion of catecholamines from rat and guinea-pig adrenal glands in potassium-free medium or after ouabain. J Physiol. 1981;313:481–498. doi: 10.1113/jphysiol.1981.sp013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Effects of desipramine, trifluoperazine and other inhibitors of calmodulin on the secretion of catecholamines from the adrenal medulla and postganglionic sympathetic nerves of the salivary gland. Naunyn Schmiedebergs Arch Pharmacol. 1984 Apr;325(4):320–327. doi: 10.1007/BF00504376. [DOI] [PubMed] [Google Scholar]


